Higher amounts of DNA methylation (hypermethylation), like that found by the researchers in some tumor DNA, decreases a gene's activity. Based on this advance, the researchers hope to spur development of a blood test that can be used to diagnose a variety of cancers at early stages, when treatments can be most effective. The study appeared February 5, 2016, in The Journal of Molecular Diagnostics.
"Finding a distinctive methylation-based signature is like looking for a spruce tree in a pine forest," said Laura Elnitski, Ph.D., a computational biologist in the Division of Intramural Research at NIH's National Human Genome Research Institute (NHGRI). "It's a technical challenge to identify, but we found an elevated methylation signature around the gene known as ZNF154 that is unique to tumors." Dr. Elnitski is head of the Genomic Functional Analysis Section and senior investigator in the Translational and Functional Genomics Branch at NHGRI.
In 2013, her research group discovered a methylation mark (or signature) around ZNF154 in 15 tumor types in 13 different organs and deemed it a possible universal cancer biomarker. Biomarkers are biological molecules that indicate the presence of disease. Dr. Elnitski's group identified the methylation mark using DNA taken from solid tumors.
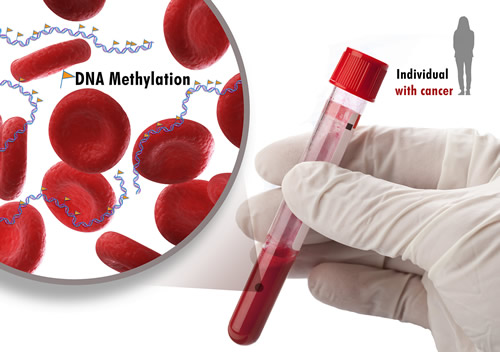
"No one in my group slept the night after that discovery," Dr. Elnitski said. "We were so excited when we found this candidate biomarker. It's the first of its kind to apply to so many types of cancer."
In this new study, they developed a series of steps that uncovered telltale methylation marks in colon, lung, breast, stomach and endometrial cancers. They showed that all the tumor types and subtypes consistently produced the same methylation mark around ZNF154.
"Finding the methylation signature was an incredibly arduous and valuable process," said NHGRI Scientific Director Dan Kastner, M.D., Ph.D. "These findings could be an important step in developing a test to identify early cancers through a blood test."
The NIH Intramural Sequencing Center sequenced the tumor DNA that had been amplified using a technique called polymerase chain reaction (PCR). Dr. Elnitski and her group then analyzed the results, finding elevated levels of methylation at ZNF154 across the different tumor types.
To verify the connection between increased methylation and cancer, Dr. Elnitski's group developed a computer program that looked at the methylation marks in the DNA of people with and without cancer. By feeding this information into the program, they were able to predict a threshold for detecting tumor DNA. Even when they reduced the amount of methylated molecules by 99 percent, the computer could still detect the cancer-related methylation marks in the mixture. Knowing that tumors often shed DNA into the bloodstream, they calculated the proportions of circulating tumor DNA that could be found in the blood.
Next steps
Dr. Elnitski will next begin screening blood samples from patients with bladder, breast, colon, pancreatic and prostate cancers to determine the accuracy of detection at low levels of circulating DNA. Tumor DNA in a person with cancer typically comprises between 1 and 10 percent of all DNA circulating in the bloodstream. The group noted that when 10 percent of the circulating DNA contains the tumor signature, their detection rate is quite good. Because the methylation could be detected at such low levels, it should be adequate to detect advanced cancer as well as some intermediate and early tumors, depending on the type.
Dr. Elnitski's group will also collaborate with Christina Annunziata, M.D., Ph.D., an investigator in the Women's Malignancies Branch and head of the Translational Genomics Section at NIH's National Cancer Institute (NCI). They will test blood samples from women with ovarian cancer to validate the process over the course of treatment and to determine if this type of analysis leads to improved detection of a recurrence and, ultimately, improved outcomes.
"Ovarian cancer is difficult to detect in its early stages, and there are no proven early detection methods," said Dr. Annunziata. "We need a reliable biomarker for detecting the disease when a cure is more likely. We are looking forward to testing Dr. Elnitski's novel approach using DNA methylation signatures."
Current blood tests are specific to a known tumor type. In other words, clinicians must first find the tumor, remove a sample of it and determine its genome sequence. Once the tumor-specific mutations are known, they can be tracked for appearance in the blood. The potential of the new approach is that no prior knowledge of cancer is required, it would be less intrusive than other screening approaches like colonoscopies and mammograms and it could be used to follow individuals at high risk for cancer or to monitor the activity of a tumor during treatment. Once the blood test is developed, the scientific community must conduct studies to ensure that it does not indicate the presence of cancer when it is not there or miss cancer when it is there.
Dr. Elnitski does not yet understand the connection between tumors and elevated DNA methylation. It may represent derailment of normal processes in the cell, or it may have something to do with the fact that tumors consume a lot of energy and circumvent the cellular processes that keep growth in check. Researchers also don't know exactly what the gene ZNF154 does.
"We have laid the groundwork for developing a diagnostic test, which offers the hope of catching cancer earlier and dramatically improving the survival rate of people with many types of cancer," Dr. Elnitski said.
NHGRI is one of the 27 institutes and centers at the NIH, an agency of the Department of Health and Human Services. The NHGRI Division of Intramural Research develops and implements technology to understand, diagnose and treat genomic and genetic diseases. Additional information about NHGRI can be found at: http://www.genome.gov.
The National Cancer Institute leads the National Cancer Program and the NIH's efforts to dramatically reduce the prevalence of cancer and improve the lives of cancer patients and their families, through research into prevention and cancer biology, the development of new interventions, and the training and mentoring of new researchers. For more information about cancer, please visit the NCI website at http://www.cancer.gov or call NCI's Cancer Information Service at 1-800-4-CANCER.
National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 institutes and centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical and translational medical research, and is investigating the causes, treatments and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.