The specific goals of UDN are to: (1) improve the level of diagnosis and care for patients with undiagnosed diseases through the development of common protocols designed by a large community of investigators; (2) facilitate research into the etiology of undiagnosed diseases, by collecting and sharing standardized, high-quality clinical and laboratory data including genotyping, phenotyping, and documentation of environmental exposures; and (3) create an integrated and collaborative community across multiple clinical sites and among laboratory and clinical investigators prepared to investigate the pathophysiology of these new and rare diseases.
In 2008, the NIH Undiagnosed Diseases Program (UDP) was organized and established by the National Human Genome Research Institute (NHGRI), the National Institutes of Health (NIH) Office of Rare Diseases Research (ORDR) and the NIH Clinical Center to help provide diagnosis and treatment for patients with unknown disorders.
In 2012, building on the early successes of the UDP, NIH extended the program into a network of seven clinical sites. These clinical sites together with a UDN Coordinating Center and other Core Laboratories comprised the Undiagnosed Diseases Network (UDN) in Phase I and demonstrated that this type of cross-disciplinary approach to disease diagnosis is feasible to implement in academic medical centers around the United States.
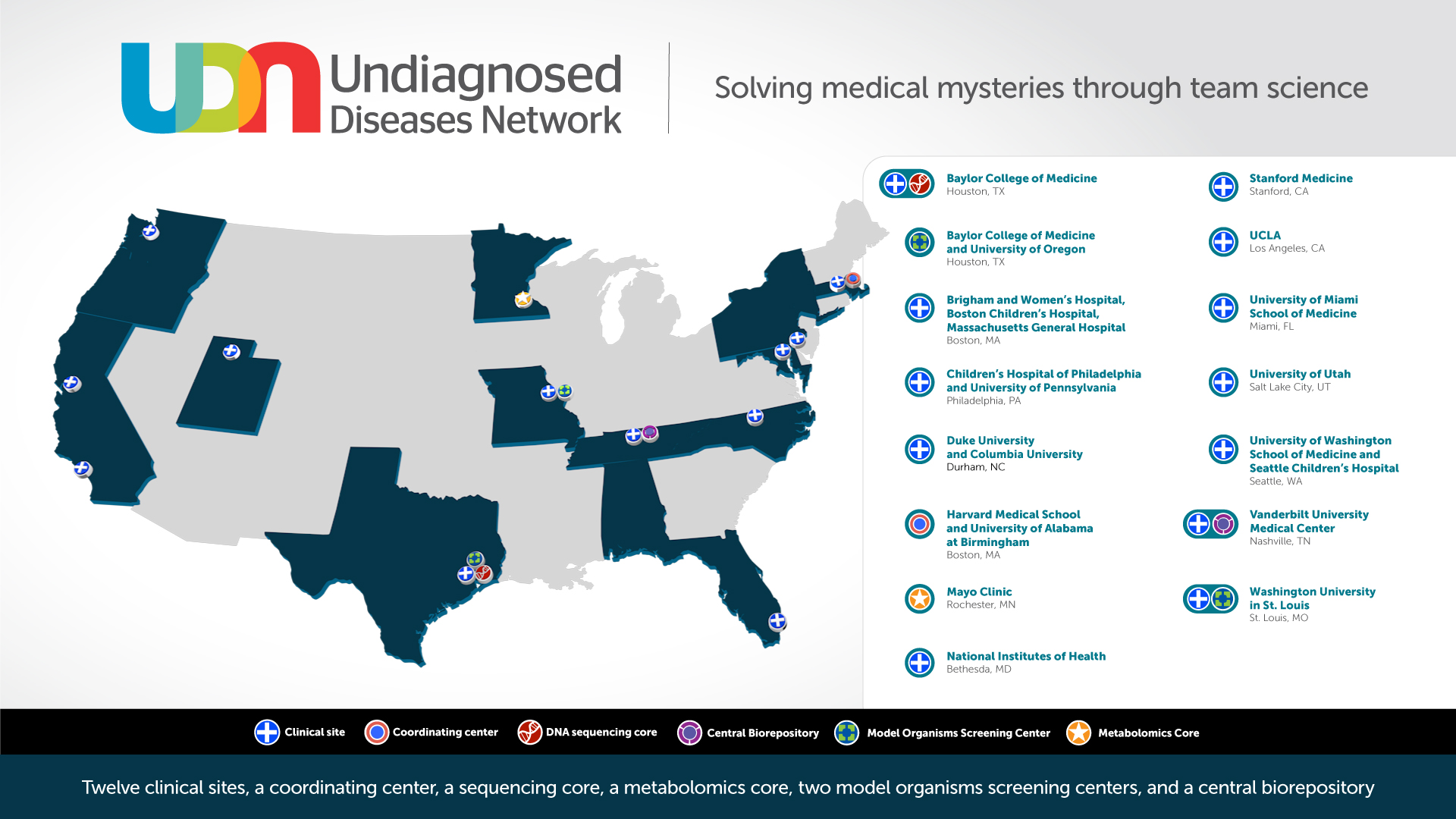
In 2018, new awards expanded the UDN from seven to 12 clinical sites, increasing the geographical distribution of the nationwide network and the number of people with access to a UDN clinical site. In addition to the new clinical sites, new research cores were also added as part of Phase II of the UDN including a new Metabolomics Core providing untargeted metabolomics and targeted biomarker analyses, and a new Model Organisms Screening Center increasing the zebrafish modeling capacity and adding C. elegans as a new model system for the UDN. See a complete list of funded awards.
NIH is using a NIH-wide approach to support the UDN beyond 2023. The National Institute of Neurological Disorders and Stroke (NINDS) is overseeing the Network in Phase III, launched in July 2023, with help from 17 different NIH Institutes and Centers along with the NIH Office of the Director. With help from patients, family members, patient advocacy groups, a Data Management and Coordinating Center (DMCC), several Clinical Sites, and other stakeholders, the NIH envisions the UDN evolving into a larger, diverse, and self-sustained network that fosters scientific discovery and provides expert diagnostic services for undiagnosed patients across the nation.
In 2023, NINDS and 17 other NIH Institutes joined together to launch the Phase III Network as a Trans-NIH Initiative. NIH has awarded a cooperative agreement to establish a new UDN Data Management and Coordinating Center (DMCC), which will provide infrastructure and research support for a new network of clinical sites. The DMCC will also support Research Cores (genomic sequencing, model organisms screening center, metabolomic and proteomic analysis, etc.) based on the needs of the Network cases, via subawards. To ease the transition from Phase II to Phase III and ensure that applicants continue to have the opportunity to receive a diagnosis, eligible Phase II extramural clinical sites were also awarded an additional year of support under the Limited Competition for the Continuation of Clinical Sites for the Undiagnosed Diseases Network Notice of Funding Opportunity (NOFO). The intramural Undiagnosed Disease Program, housed within the NIH Clinical Center and currently supported as a UDN clinical site, will continue to receive support and oversight from multiple NIH Institutes and Centers.