Last updated: April 01, 2006
Promoting Safe and Effective Genetic Testing in the United States - Chapter 4
Promoting Safe and Effective Genetic Testing in the United States
Chapter 4
Improving Providers' Understanding of Genetic Testing
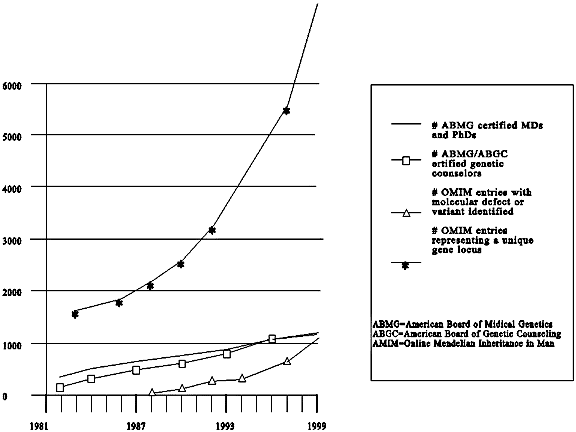
The increase in the number of disease-related genes that scientists have identified in recent years, particularly those in which inherited mutations increase susceptibility to common disorders, has engendered expectations that health care will be improved. The rate of increase of health care professionals trained and board-certified in medical genetics or genetic counseling has not kept pace with the rate of increase of genetic discovery and of potential demand for genetic tests. Although genetic professionals currently in practice or in training could meet a small increase in demand for genetic testing and counseling, their supply is insufficient to cope with even a doubling of the demand. Some commentators maintain that population carrier screening for just one condition, cystic fibrosis, would swamp the system.1 Thus, if the demand for genetic testing increases, and the supply of genetics providers does not keep pace, other health care professionals will have to play a role, or new models of testing will have to be devised if the demands are to be met. In this chapter, we first delineate a role for non-genetic health care professionals in eliciting genetic risks and providing genetic tests. We then turn to the obstacles of having non-geneticists provide these services. We next consider policies for overcoming the obstacles and, finally, other models for providing genetic services.
A ROLE FOR NON-GENETIC HEALTH CARE PROFESSIONALS
In addition to the paucity of genetic specialists relative to the potential demand for genetic testing, there are other reasons why other professionals should be involved in genetic testing. First, few people have sufficient understanding of genetics to recognize whether or not they or their children are at increased risk of inherited disease. Therefore, health care professionals who provide care to most people have a responsibility to determine whether a high-risk situation is present. With the rise in managed care in the United States, these professionals are increasingly primary care providers who provide first-contact and continuing care and who may serve as gatekeepers for access to other specialists. Nevertheless, in the United States, many people can bypass primary care providers and seek care directly from specialists. Even when aware that a problem that concerns them might have a genetic origin, they are more likely to seek the care of the specialist who manages the problem when it becomes overt than the care of a geneticist. For instance, people concerned about an inherited susceptibility to cancer will go to an oncologist or surgeon more often than to a geneticist, and pregnant women concerned about birth defects or inherited disorders will ask their obstetrician instead of a geneticist. Consequently, non-genetic specialists, as well as primary care providers, become the gateway to genetic testing.
Second, primary care and other providers that people visit periodically are in an excellent position to elicit risk information. One important source of information about genetic risks is family history. When people receive their care from one source over a period of years, as is the ideal primary care situation, the provider is more likely to learn about family history as relatives become ill (whether they are in the provider's care or not) and, possibly, about other situations that raise the risks of genetic disease. If the source remains constant but the providers change, a single medical record used by all of the patient's providers gives the current provider an opportunity to recognize risk factors, if the record is adequate. This advantage is lost when people change their source of care (at least until a universal medical record that people keep with them, such as a "smart card", is developed). Each new provider, including specialists, must attempt to ferret risk factors, including family history, again. The few studies that have been done show that family history, as elicited directly from people, does not always accurately reflect what medical records of relatives contain.2-4 Despite their skill and expertise, genetic specialists who see a person only once, as is often the case in prenatal care, might not be able to elicit as complete a picture of risk factors for genetic disease as the primary care provider who sees the person repeatedly. Moreover, without recognition of genetic risk factors by primary care providers or other non-genetic health care providers, many people will never get to a genetic specialist.
Eliciting Risks of Genetic Disease in Healthy People
Family History. Although family history is an important source of information about risks of future genetic disease, it has limitations. We have already mentioned the problem of reliability. More importantly, its yield will depend on the mode of inheritance of the diseases of concern. It is most useful when diseases are inherited in a dominant or X-linked fashion. Some diseases inherited in these ways will, however, arise by new mutation and the family history will be negative. Eliciting a history of frequently-recurring common diseases that do not follow Mendelian inheritance might indicate the presence of inherited susceptibility, such as those for breast and colon cancer, or of polymorphisms that have been associated with disease. The family history is less likely to be informative for autosomal recessive diseases in which each parent of an affected child is an asymptomatic carrier. Eliciting a history of consanguinity in the parents points to an increased risk of autosomal recessive diseases in their children; the parents might each have inherited the same disease-related alleles from a common ancestor. This also explains why some autosomal diseases are higher in certain ethnic groups in the absence of consanguinity. Thus eliciting a person's ethnicity also becomes important. When people have many children, it is more likely that the family history will be positive for a recessive disease; on average, one out of four children will be affected. Adoption, the use of artificial insemination by donor sperm and multiple sexual partners, as well as people's greater mobility (removing them from the nuclear family), increase the difficulty in eliciting an informative family history. One systematic method of collecting family history data, and also establishing whether consanguinity is present, is the construction of a pedigree.
Past History. In view of the limitation of family history and ethnic origin, the health care provider must look for other ways of determining genetic risk factors for future disease. Some risk factors can also be elicited by interview. These include (1) the age of a pregnant woman; as maternal age increases, particularly over 35 years, the risk of Down syndrome and other chromosomal abnormalities in her fetus increases, and (2) past or present exposure to an environment that is more likely to result in disease in those with genetic predispositions, such as intake of fava beans or anti-malarial drugs in people with glucose-6-phosphate dehydrogenase deficiency, which has a higher frequency in people of African, Asian, and Mediterranean origin.
Genetic Testing. Finally, genetic testing can be used to elicit risks of future genetic disease. If the person's history is unrevealing and if the disease is a serious one that can be avoided by reproductive options, prevented, or more effectively treated by intervention in its presymptomatic stages than after symptoms appear, then population-wide screening can reduce the burden of the disease if it is utilized by many in the population. In the absence of an affected family member, carriers for autosomal recessive disease can be detected by genetic screening. Genetic testing can also confirm the presence of specific disease-related alleles in people with positive histories, pointing the way to specific interventions. Testing and screening should only be undertaken in clinical practice when the conditions for testing described in Chapter 2 have been satisfied.
The question can be asked, why not simply screen everyone for disease-related alleles and bypass the family and past history? First, relatively few predictive tests applied on a population basis meet the criteria of validity and utility described in Chapter 2. Second, even if they did, it would be extremely costly to test everyone. As the cost of the technology is reduced, this reason becomes less important. Third, the process of offering predictive genetic screening takes time. In accord with the principles of autonomy presented in Chapter 1, people must be informed of the benefits and risks of screening and given an opportunity to decline it. Although this might be accomplished simply by brochures and other audio-visual aids, the effectiveness of these methods has not yet been established. Unless and until they are, providers will have to spend time explaining screening to potential users. Fourth, when the results come back, they have to be interpreted. As discussed in Chapter 2, many genetic tests are not perfect predictors. The probability that disease will occur when the test result is negative, or that disease will not occur when the result is positive, both of which will be greater when populations rather than at-risk individuals are tested, must be explained.
The Role of Non-genetic Health Care Providers
With proper training and adequate knowledge of test validity, disease and mutation frequencies in the ethnic groups to whom they provide care, primary care providers and other non-genetic specialists can and should be the ones to offer predictive genetic tests to at-risk individuals. In some circumstances, for instance, when the family history is complicated or the symptomatology in relatives does not point to a clear diagnosis, referral to a genetic specialist is appropriate before offering testing. Unless there are other means of providing screening, such as through hospitals (for newborn screening) or public health facilities (see section on Other Models later in this chapter), non-genetic providers will almost always be involved in offering genetic screening, as well as testing. The role of non-genetic providers in interpreting test results is complex. The interpretation of positive results will often depend on further elicitation of risks, including family history. The options available to reduce risks will also have to be considered. Positive results can have implications for future children. Often they will also be of importance to other relatives with whom the person tested should be encouraged to communicate. For tests with imperfect sensitivity and those for susceptibility to common disorders, negative results do not eliminate the chance of future disease. A test's sensitivity and predictive value can also vary by ethnic group (e.g., the sensitivity of current CF carrier tests is much higher in Caucasians than in African or Asian Americans). Providers must be aware of these and other considerations in interpreting test results, and be capable of communicating risk information and its implications to those who are tested or their parents or guardians. Consultation with geneticists and/or genetic counselors might be appropriate.
OBSTACLES TO THE INVOLVEMENT OF NON-GENETIC PROFESSIONALS
Despite the advantages of non-genetic providers being the gateway to genetic testing, there are drawbacks. One is the limited knowledge of genetics and genetic tests of some non-geneticist providers. In a 1991 survey of physicians selected at random from ten states, non-genetic, non-academic physicians in five specialties (family practice, internal medicine, obstetrics-gynecology, pediatrics, and psychiatry) were able to correctly answer an average of 73.1 percent of questions deemed important by a panel of non-genetic providers who helped develop the questionnaire. Physicians who graduated from medical school between 1971 and 1985 scored significantly higher than those who graduated between 1950 and 1970. Having a genetics course in medical school was significantly associated with higher scores but was not as important a predictor as the year of graduation. Physicians in specialties that had been exposed to genetic problems in their practices (family physicians who delivered babies, pediatricians, and obstetrician-gynecologists) had significantly higher scores than physicians in the other specialties. Over one-third of family physicians who did not deliver babies, internists, and psychiatrists had scores of 65 percent correct or lower.6
In a 1996 survey on testing for genetic susceptibility to cancer, Burke and Press found that of the first 124 primary care physicians to respond, over 20 percent had not heard of a test for a genetic predisposition to breast cancer. (N. Press, personal communication)
Another drawback is the tendency of non-geneticist providers to be directive in situations in which reproductive options to avoid the conception or birth of an infant with a serious disorder are considered.7-11 Primary care providers occasionally report that they will not offer a prenatal test to a patient who they are confident would not be interested in testing.8 Whether the provider, even one with a continuing relationship with the patient, really does know the patient's attitudes on this subject and, if so, is justified in withholding information, is debatable. Recently, it has been recognized that nondirectiveness might not be achievable and might not be something that patients always want.12-15 Nevertheless, because of past efforts to deny people the opportunity to reproduce because they possessed presumably heritable traits,16,17 and the need to respect personal autonomy in reproductive matters, efforts to steer people toward a particular reproductive decision are undesirable (see Chapter 1).
When safe, effective, and widely acceptable interventions are available for people with positive predictive test results, the role of nondirectiveness is much less of an issue. When interventions are not of proven safety and effectiveness, people should be told that is the case and should decide for themselves whether they want testing and, if they do and subsequently have a positive test result, whether they want the unproven intervention.
It is not clear that primary care providers could devote the time that informing patients about risks and benefits of genetic tests often entails. The average time spent counseling new patients in genetics or prenatal clinics exceeds 1 hour.18 The median time of counseling for molecular genetic testing is 1 hour, not counting preparation (record review) or clerical and administrative time.19
POLICIES FOR IMPROVING THE ABILITY OF NON-GENETIC HEALTH CARE PROFESSIONALS TO BE INVOLVED IN GENETIC TESTING
The Task Force considered a number of strategies, both long and short-term, for improving the ability of non-genetic health care professionals to provide genetic services safely and effectively.
Greater Public Knowledge of Genetics
A knowledge base on genetics and genetic testing should be developed for the general public. Without a sound knowledge base, informed decisions are impossible and claims of autonomy and informed consent suspect. People who are more knowledgeable will grasp more readily the issues raised by providers when they offer tests. This could diminish the time needed for education and counseling without reducing consideration of the implications of testing. Policies for improving public understanding of genetics and genetic tests are beyond the scope of the Task Force. A number of private and public organizations have, through public statement and program investment, strongly endorsed the need for large-scale educational programs.a Educating the public in genetics presents enormous challenges. Many people's views of how traits are inherited are inconsistent with Mendelian inheritance.20 New models of providing education and counseling to patients and other consumers are needed.
Ethnic groups differ in their perceptions of disease origins and what should be done to avert disease.21-23 Moreover, identifying a genetic variant that has a much higher frequency in some ethnic groups than in others could have a stigmatizing effect on that group. In keeping with the overarching principles described in Chapter 1, sensitivity to cultural differences is of paramount importance. Unfortunately, minorities are seriously under-represented in the field of genetics.
Professional Education
Undergraduate and Graduate Medical Education. The Task Force encourages the development of genetics curricula in medical school and residency training to enable all physicians to recognize inherited risk factors in patients and families, and appreciate issues in genetic testing and the use of genetic services. A committee of the American Society of Human Genetics has published a list of objectives for medical school courses and the skills and attitudes they should engender in medical students.24
According to a 1995 survey by the Association of American Medical Colleges (AAMC), 68 of 125 four-year medical schools in the U.S. required genetics courses in their curricula (personal communication from Al Salas, Association of American Medical Colleges to Task Force, July 16, 1997). Although genetics is sometimes an integral part of other basic science courses in some other medical schools, the Task Force is concerned that genetics is not being taught adequately to all medical students. The AAMC survey also found that most genetics is taught in the first 2 (basic science) years of medical school. Consequently, many clinical aspects will not receive adequate attention. The Task Force is not suggesting that the courses be moved to the clinical years but that clinical departments pay greater attention to genetic issues.
As provider-patient communication is critical in offering genetic tests and counseling about them, consumers should be involved in the planning and implementation of new curricula in genetics. The Partnership for Genetic Services Pilot Program, just launched by the Alliance of Genetic Support Groups, and supported by public and private funds, has, as its goal, improving medical student and provider understanding, sensitivity, and competence in delivering genetic services. It will do this by exposing medical students and physicians-in-training to relevant community resource systems and illustrative presentations by consumers. Partnerships between consumers and clinical genetics providers, primary care practitioners, medical school faculty, and managed care administrators have been established.
Licensure and Certification. The likelihood that genetics will be covered in curricula will improve if relevant genetics questions are included in general licensure and specialty board certification examinations, and if correctly answering a proportion of the genetics questions is needed to attain a passing score. Medical school curriculum and residency review committees, which exist at both the local medical school and hospital levels and at the national level, define teaching content based on core material needed for clinical practice, recent advances, and questions on board examinations. Those who prepare board examinations, the National Board of Medical Examiners for medical students, and the various specialty boards for specialty certification, derive questions from material they think important, yet questions involving genetics are sparse and sometimes inappropriate. The American Council of Graduate and Medical Education (ACGME), is the umbrella organization for boards and residency review committees, and also contributes importantly to residency training content. The Task Force encourages ACGME, as well as residency review committees, to consider the importance of graduate training in genetics. The Task Force is pleased that the American Board of Obstetrics and Gynecology and the Society of Perinatal Obstetricians have acknowledged the importance of teaching genetics, including ethical aspects, by including questions on the basic obstetrics and gynecology exams, as well as on the subspecialty board exams of Maternal-Fetal Medicine.b
Traditionally, much medical school education and residency training occurred on the wards of hospitals. Those responsible for education and training have begun to recognize that most medical care is provided in ambulatory settings and that the delivery of care in those areas presents challenges for education. Genetic testing is a prime example. Moreover, teaching about genetic tests, including such issues as analytic and clinical validity, introduces students and residents to general problems of reliability and test sensitivity and specificity, which are important for a much wider range of clinical laboratory tests.
In a rapidly changing field such as genetics, curricula that focus on current discoveries and do not lay a basic framework will rapidly become obsolete. The Task Force is particularly concerned that underlying concepts of genetics are not adequately learned by all physicians.25 Equally important are the means of communicating genetic concepts and risks to patients. Although the tests will change, many aspects of patient-provider communication will not, although here, too, much research is beginning to explore the nature of these interactions.
Continuing Medical Education. The full beneficial effects of improving medical school and residency curricula in genetics will not be felt for many years. Consequently, improving the ability of providers currently in practice to offer and interpret genetic tests correctly is of paramount importance. The Task Force vigorously debated the question of whether this goal could best be accomplished by a "carrot" or "stick" approach. An early position taken by the Task Force was: "Some documentation of continuing education in the area of human and medical genetics should be required for physicians offering genetic tests, including primary care providers."26 (p.24) As the Task Force deliberated, it developed doubts as to whether continuing education could accomplish this goal and whether a requirement would accomplish the Task Force's objective. The Task Force was also concerned that such a requirement would be difficult to enforce. The need to demonstrate competence is discussed further in the next section, but the point the Task Force wishes to emphasize is the need for each specialty to recognize that all of those who are certified in that specialty appreciate the importance of genetics and genetic tests relevant to that specialty. In addition to basic curricula already considered, the Task Force recommends that each specialty involved with the care of patients with disorders with genetic components should design its own curriculum for continuing education in genetics.
Administrators and other nonphysician personnel who triage patients and/or make coverage or reimbursement decisions, such as those in managed care organizations, should also have knowledge of the benefits and risks of genetic testing.
Demonstrating Provider Competence
Hospitals and managed care organizations, on advice from the relevant medical specialty departments, should require evidence of competence before permitting providers to order predictive genetic tests defined as needing stringent scrutiny or to counsel about them. Periodic, systematic medical record review, with feedback to providers, should also be used to ensure appropriate use of genetic tests.
Prerequisites. If hospitals and managed care organizations are to require evidence of competence, three prerequisites must be met. First, a mechanism must be in place for deciding which tests need evidence of competence. The Task Force believes that this should be one of the tasks of the proposed Secretary's Advisory Committee described in Chapter 1. Second, competence must be defined. This can be accomplished by agreement between representatives of the non-genetic specialties involved in testing and of the genetics profession. Guidelines for establishing competence developed at the national level, e.g., by professional societies, which could be facilitated by the proposed Secretary's Advisory Committee, are ultimately preferable, but local agreements might be more readily reached when a test first becomes available. Third, easily accessible educational modules must be available to enable providers to gain competence. (We discuss some possibilities in the next section.) Unless continuing education opportunities are readily available, providers will be deterred from gaining sufficient knowledge of genetics to enable them to offer genetic tests appropriately to their patients.
Although little precedent exists for asking for a demonstration of competence before ordering tests that will be performed primarily in ambulatory settings, there are several reasons why some predictive genetic tests (those requiring stringent scrutiny) should be ordered only by those with demonstrated competence. Some of these reasons are important primarily to the person being tested, some to the provider offering the test, and some to those paying for the test.
- People need to have sufficient information about the clinical validity of the test to decide whether the test is appropriate for them. Providers must be able to give them the requisite information.
- The implications of a positive or negative test result might influence people's decision to be tested. Providers must be aware of the implications and discuss them with the people considering testing.
- People's autonomy must be respected especially when procedures for avoiding the conception or birth of a child with a genetic disease are options following a positive test result. Autonomy is also crucial when the interventions in those with positive test results have not been proven to be safe and effective. Providers must recognize these situations, understand the need to respect autonomy, and be able to communicate information in the least directive manner possible.
- The results of some predictive genetic tests will indicate that relatives might be at risk of genetic disease. Providers must be prepared to discuss why and how the person tested should communicate with relatives and what the relatives should do.
- Providers could face legal liability if they order a test inappropriately or if they communicate results to relatives (except in extreme circumstances - see Chapter 1) or unrelated third parties without the consent of the person tested.
- Third parties paying for the test, including managed care organizations, will not want to reimburse if the test has been ordered unnecessarily or inappropriately.
Enforcement. The Task Force does not favor requiring organizations to establish competence requirements. It believes self-interest, as just discussed, will lead many organizations to set them. Nor is it necessary for laboratories to request documentation of competence before they will perform a genetic test. Providers who work for organizations who do credential for genetic testing would place themselves in legal jeopardy if they ordered the test without having the credential. Providers who do not work with an organization that credentials, for instance a solo practitioner in private practice, might be competent to order genetic tests but will have no credential to present. In their survey, Burke and Press found that one quarter of respondents disagreed strongly with a suggestion that physicians should be required to undergo a brief certification in genetics before they could order susceptibility tests; less than 19 percent agreed strongly. The majority expressed moderate support for this position. (N. Press, personal communication, June 1997) As discussed in Chapter 3, the laboratory does have a responsibility to determine from the requisition that the test is indicated, and that, when appropriate, informed consent has been obtained from the person to be tested or his or her legal guardian.
Medical record audits assure managed care and other organizations that providers are satisfying standards of care. The feedback given to providers also serves as a valuable reenforcement to what has previously been learned. Audits of records for frequently-ordered medical tests should be considered. The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and the National Committee for Quality Assurance (NCQA) should consider asking hospitals and other health care organizations to develop continuous quality improvement programs focusing on genetic testing.
Assisting Providers in Gaining Competence in Genetics
When organizations begin to require that providers have demonstrable competence in genetics, the means of acquiring that competence must be available. The American College of Medical Genetics is working with other specialties to set guidelines and standards to assist in the development of curricula. It responded to a request from the American Society of Clinical Oncologists to assist it setting up "train the trainer" modules for oncologists who can then train others in their specialty. ACMG would be responsive to requests from other organizations as well.
The Task Force endorses the recent establishment of a National Coalition for Health Professional Education in Genetics (NCHPEG) by the American Medical Association, the American Nurses Association, and the National Human Genome Research Institute. The Coalition should work in consultation with non-genetic professional societies, such as the Association of American Medical Colleges, the American Council on Graduate and Medical Education, and genetic societies, such as the American College of Medical Genetics, the National Society of Genetic Counselors, the International Society of Nurses in Genetics, and appropriate consumer groups to encourage the development of core curricula in genetics. It should encourage input by consumers in the development of these curricula. In order to avoid duplication, the Coalition should serve as a registry and clearinghouse for, and disseminator of, information about various curricula and educational programs, grants, and training pilot programs in genetics education. It should encourage professional societies to track the effectiveness of their respective educational programs.
The Task Force welcomes the interest of the Agency for Health Care Policy and Research (AHCPR) in helping the Coalition develop a research agenda in health education.
In 1994, the Maternal and Child Health Bureau (MCHB) of the Health Resources and Services Administration, through its Genetic Services Branch, began soliciting grant applications to strengthen genetics in primary care. Thus far nine programs have been funded and several more are expected to be funded in 1998.c At least one educational module is available on the World Wide Web under MCH NetLink. Others will appear shortly. Another MCHB grantee, the Council of Regional Networks for Genetic Services (CORN) has recently issued its Guidelines for Clinical Genetic Services for the Public's Health.26 MCHB has also asked CORN to prepare national guidelines that can be used for comprehensive followup care of children and families with rare metabolic disorders that can be used by purchasers and service providers in negotiating contracts with managed care plans. CDC has developed a Public Health Training Network that can be adapted to provide information about genetics. The network often employs satellite broadcasting to multiple receiving sites with phone communication from the sites to permit two-way communication. The Network also develops material for Internet presentations and self-study, computer-based training modules. A wide range of subjects have been presented, including basic epidemiology, specific disease management, immunizations, and managing laboratories under CLIA. The format includes lectures, panel discussions, and videos.
A major problem in all educational endeavors is finding the "teachable moment," the time at which people, including health care providers, are receptive to new information and are most likely to retain it. These moments arise when providers are asked questions about genetic tests or when charts are flagged because the patient fulfills criteria for being offered a genetic test. Clearly, more people are asking providers questions about genetic tests. Computerized medical records or self-completing questionnaires (see box entitled Educational Module to Assist Physicians in Recognizing Genetic Risks) can generate flags to advise providers to offer genetic tests to people at risk. Printouts of background information, when flags are raised, could assist the provider. A 1-800 hotline that providers (and the public, perhaps,) can call to learn more about specific genetic tests, including availability and indications for their use, should be established by NCHPEG or some of its governmental and private constituent organizations.
OTHER MODELS
Nursing
The overall time that physicians spend talking with patients on all subjects is usually less than the time that genetic counselors spend informing people about genetic tests and their implications. The nursing profession has recognized that nurses have much to offer in helping people appreciate the benefits and risks of genetic testing.27,28 Nurses can not only counsel (when trained) but also perform a wide range of activities in health care that genetic counselors are not qualified to do. Nurses are also in much greater supply. Nurses have been shown to be effective in providing education for testing for genetic susceptibility to cancer.29 Oncology nurses increasingly view themselves as genetic health care professionals.d One nursing organization told the Task Force, "We see genetic education as core content in nursing education at both the undergraduate and advanced levels."e Nurses have played a large role in genetic counseling in the United Kingdom for many years.30 A study currently under way in the U.S. is comparing the ability of nurse practitioners and genetic counselors in educating and counseling about testing for genetic susceptibility to breast cancer. (G. Geller, work in progress) Nurses should be provided with additional education and training that can increase their effectiveness in providing education for people undergoing genetic testing.
Community and Public Health
Although population-wide screening can be integrated into personal health care - prenatal screening in obstetrics provides a good example--different models have been used. In each case, screening has been undertaken because it permitted detection of many more at-risk subjects than would have been possible by using family histories. In this way, the opportunities for avoidance, prevention, or effective treatment are greater than waiting for symptoms to appear. For instance, diagnosing an older infant or child with phenylketonuria does little to prevent her retardation although it alerts the family to its risk of having additional children with that disease. Newborn screening, on the other hand, prevents retardation of the first child and all others who carry the disease-causing genotype (see Appendix 5).
In many states, it is the responsibility of the hospital in which the baby is born to conduct screening. This model takes advantage of the fact that most babies are born in the hospital, making it easy to reach them. It is not advantageous once babies are discharged. Nevertheless, as testing for more inherited conditions become available and the safety and effectiveness of treating them neonatally is established, newborn screening could expand markedly.
Community-centered screening presents another model. Tay-Sachs carrier screening was originally organized at the community level; health care professionals who staffed the sites generally volunteered their time. The success of this effort depended on the cooperation of a cohesive community committed to screening. Nevertheless, not everyone in the ethnic group at risk came for screening and other methods had to be devised to reach them (see Appendix 6). In the 1970s, and to a lesser extent today, sickle cell screening was performed at community sites and in health department clinics. For reasons discussed in Appendix 6, this screening was not always a great success. Today, screening newborns for sickle cell anemia is part of many States' newborn screening programs.31 Sickle cell screening is succeeding in lowering morbidity and mortality from this disease among African-American children.32 Any effort to initiate community-based genetic screening must have the active support of the community. Particularly when minority communities are involved, the program must be sensitive to issues of discrimination and provide sufficient resources for education and counseling.
Many other disorders are spread throughout diverse communities and it would be a Herculean task to organize community-based screening. Screening could be offered in health department clinics, mobile vans, or other sites, but not all segments of the population are likely to utilize them. A greater chance of breaching confidentiality is possible at community and health department sites than in the privacy of the traditional provider-patient relationship. Informed consent might not always be obtained.33 Traditionally, health departments have been most involved in clinical care when there were well-accepted interventions (such as immunizations or tuberculosis control) without which the health of the public would be jeopardized. It might be difficult for public health personnel to appreciate that someone who refuses genetic screening is not jeopardizing the health of the public. Before these new models can be investigated, additional training of the personnel involved is necessary. Schools of nursing, public health, and social work need to strengthen their training programs in genetics.f
REFERENCES
- Wilfond BS, Fost N: The cystic fibrosis gene: Medical and societal implications for heterozygote detection. JAMA 1990;263:2777-2783.
- Mendlewicz J, Fleiss JL, Cataldo M, Rainer JD: Accuracy of the family history method in affective illness. Comparison with direct interviews in family studies. Archives of General Psychiatry 1975;32:309-314.
- Kee F, Tiret L, Robo JY, et al: Reliability of reported family history of myocardial infarction. BMJ 1993;307:1528-1530.
- Offit K, Brown K: Quantitating familial cancer risk: A resource for clinical oncologists. Journal of Clinical Oncology 1994;12:1724-1736.
- Giardiello FM, Brensinger JD, Petersen GM, et al: The use and interpretation of commercial APC gene testing for familial adenomatous polyposis. New England Journal of Medicine 1997;336:823-827.
- Hofman KJ, Tambor ES, Chase GA, Geller G, Faden RR, Holtzman NA: Physicians' knowledge of genetics and genetic tests. Academic Medicine 1993;68:625-631.
- Geller G, Tambor ES, Chase GA, Hofman KJ, Faden RR, Holtzman NA: Incorporation of genetics in primary care practice. Will physicians do the counseling and will they be directive? Archives of Family Medicine 1993;2:1119-1125.
- Geller G, Holtzman NA: A qualitative assessment of primary care physicians' perceptions about the ethical and social implications of offering genetic tesing. Qualitative Health Research 1995;5:97-116.
- Holmes-Siedle MN, Rynanen M, Lindenbaum RH: Parental decisions regarding termination of pregnancy following prenatal detection of sex chromosome abnormality. Prenatal Diagnosis 1987;7:239-244.
- Marteau TM, Plenicar M, Kidd J: Obstetricians presenting amniocentesis to pregnant women: Practice observed. Journal of Reproductive and Infant Psychology 1993;11:3-10.
- Marteau TM, Drake H, Bobrow M: Counselling following diagnosis of a fetal abnormality: The differing approaches of obstetricians, clinical geneticists, and genetic nurses. Journal of Medical Genetics 1994;31:864-867.
- Bernhardt BA: Empirical evidence that genetic counseling is directive: Where do we go from here? American Journal of Human Genetics 1997;60:17-20.
- Michie S, Bron F, Bobrow M, Marteau TM: Nondirectiveness in genetic counseling: An empirical study. American Journal of Human Genetics 1997;60:40-47.
- Kessler S: Psychological aspects of genetic counseling. VII. Thoughts on directiveness. Journal of Genetic Counseling 1992;1:9-17.
- Clarke A: Is non-directive genetic counselling possible? The Lancet 1991;338:998-1001.
- Kevles DJ: In the name of eugenics. New York, Alfred A. Knopf Inc. 1985.
- Reilly P: The surgical solution: A history of involuntary sterilization in the United States. Baltimore, The Johns Hopkins University Press; 1991.
- Bernhardt BA, Pyertiz RE: The economics of clinical genetics services. III. Cognitive genetics services are not self-supporting. American Journal of Human Genetics 1989;44:288-293.
- Surh LC, Wright PG, Cappelli M, et al: Delivery of molecular genetic services within a health care system: Time analysis of the clinical workload. American Journal of Human Genetics 1995;56:760-768.
- Richards M: Lay and professional knowledge of genetics and inheritance. Public Understanding of Science 1996;5:217-230
- Angel R, Thoits P: The impact of culture on the cognitive structure of illness. Cultural Medical Psychiatry 1987;11:465-494.
- Punales-Morejon D, Penchaszadeh VB: Psychosocial aspects of genetic counseling: Cross-cultural issues. Birth Defects 1992;28:11-15.
- Dibble SL, Vanoni JM, Miaskowski C: Women's attitudes toward breast cancer screening procedures: Differences by ethnicity. Women's Health Issues 1997;7:47-54.
- American Society of Human Genetics Information and Education Committee: Report from the ASHG Information and Education Committee: Medical school core curriculum in genetics. American Journal of Human Genetics 1995;56:535-537.
- Task Force on Genetic Testing: Interim principles. Available at www.med.jhu.edu/tfgtelsi 1996.
- Council of Regional Networks for Genetic Services (CORN): Guidelines for Clinical Genetic Services for the Public's Health. 1997;First Edition, CORN, Atlanta GA.
- Monsen RB: Nursing takes leading role in genetics education: Coalition formed to increase provider awareness on genetics technologies. American Nurse 1996;28:11
- Anderson GW: The evolution and status of genetics education in nursing in the United States 1983-1995. Image The Journal of Nursing Scholarship 1996;28:101-106.
- Lerman C, Biesecker B, Benkendorf JL, et al: Controlled trial of pretest education approaches to enhance infomed decission-making for BRCA1. Journal of the National Cancer Institute 1997;89:148-157.
- Williams A: Genetic counseling. A nurse's perspective. In Clarke A (ed): Genetic Counseling. Practice and Principles. New York, Routledge; 1994:44-62.
- Hiller EH, Landenburger G, Natowicz MR: Public participation in medical policy making and the status of consumer autonomy: The example of newborn screening programs in the United States. American Journal of Public Health 1997;87:1280-1288.
- Vichinsky E, Hurst D, Earles A, Kleman K, Lubin B: Newborn screening for sickle cell disease: Effect on mortality. Pediatrics 1988;81:749-755.
- Farfel MR, Holtzman NA: Education, consent, and counseling in sickle cell screening programs: Report of a survey. American Journal of Public Health 1984;74:373-375
| Top of page |