Last updated: July 03, 2013
NHGRI finds \"off\" switch that underlies rare genetic disease affecting boys
NHGRI finds "off" switch that underlies rare genetic disease affecting boys
By Steve Benowitz
Special to NHGRI
 |
A rare, genetic disease found only in boys is helping researchers at the National Human Genome Research Institute (NHGRI) unlock secrets about how the body fends off infection. Studying a mouse model of an inherited disease called X-linked lymphoproliferative syndrome (XLP), they have discovered new details explaining how a missing protein can disrupt communication between two important types of white blood cells, T and B cells, which play key roles in immunity.
The findings also help explain why in some patients with XLP, miscommunication between T and B cells can be deadly, turning it into an often fatal immune disorder triggered by infection with an otherwise non-lethal, common virus, Epstein-Barr virus (EBV). The findings, Positive and Negative Signaling through SLAM Receptors Regulate Synapse Organization and Thresholds of Cytolysis, were reported in the June 7 online version of the journal Immunity.
XLP affects about one in a million boys. About half of these children experience a severe immune response to infection with EBV, resulting in symptoms that can include fever, hepatitis, an enlarged spleen, abnormally low numbers of antibodies, and, in some cases, lymphoma and other blood disorders. EBV infects B cells, and normally, the body clears such infections, but individuals with XLP are unable to kill these EBV-infected cells.
"It wasn't clear why all of these patients were dying from Epstein-Barr virus, and now we have a much better idea," said Pamela Schwartzberg, M.D., Ph.D., senior author and a senior investigator in NHGRI's Genetic Disease Research Branch (GDRB). The paper's other authors included Roseanne F. Zhao, Jennifer Cannons and Mala Dutta, also with GDRB, and Gillian M. Griffiths with the Cambridge Institute for Medical Research in the U.K. "These results teach us a whole new way of looking at how T cells and B cells interact and provide insights into how T cells regulate B cell function. This gives us a better understanding into not only the pathophysiology of the disease, but also into the basic mechanism of how we get long-term antibody immunity."
 |
T cells come in two flavors - the virus-killing version, called cytotoxic or "killer" T cells, and helper T cells, which aid in a variety of cellular functions. For example, B cells produce antibodies against infection with aid from helper T cells. Killer T cells, on the other hand, are responsible for recognizing and killing virally-infected cells. Normally, T cells kill off the EBV-infected B cells and patients recover from infection.
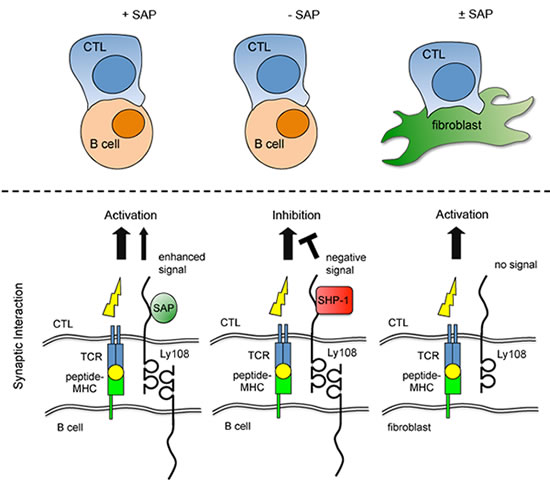
The system breaks down in XLP due to the mutation of a gene that prevents individuals from making a protein called SAP. When normal, SAP regulates communication between antibody-producing B cells and T cells, which destroy foreign invaders such as viruses.
Dr. Schwartzberg and her colleagues showed that such mutations result in a complex interplay of both so-called "positive" and "negative" signals, which interrupts an even more complicated temporary marriage between T cells and B cells. They found that in the absence of SAP, another molecule steps in and shuts down the T cell-B cell connection, preventing the immune system from preventing EBV infection.
The latest findings come on the heels of Dr. Schwartzberg's earlier work in mouse models of XLP and immunity. Dr. Schwartzberg's group, in collaboration with researchers at the National Institute of Allergy and Infectious Diseases, previously showed that the impaired immunity is caused by abnormal interactions between T and B cells. The SAP-deficient mice lacked structures called germinal centers. These are sites where B cells produce specific antibodies, and where so-called "memory B cells" are made for long-term immunity.
"Since the T cells couldn't stick well to B cells, and the major defect is that XLP patients can't kill EBV-infected B cells, we thought that this inability to stick together could be a major feature of the disease. The helper T cells can't help the B cells make antibodies, and the killer T cells that normally kill off infected B cells can't kill them," Dr. Schwartzberg said.
Using a combination of special microscopy to visualize cell interactions, along with detailed biochemistry, Dr. Schwartzberg's team subsequently acquired an integrated image of why these animals don't respond properly to infection. Investigators showed that the killer T cells don't react properly to signals on B cell surfaces in SAP-deficient mice. They also can't kill B cell targets, though the T cells can kill any other type of infected cells. Normally, SAP binds to a family of cell surface receptors.
More specifically, an organized structure called the "immune synapse" forms where T and B cells interact. In XLP, T cells - both helper and killer - can't interact with B cells in a stable way because they lack SAP, which is a crucial link in a series of biochemical messages. They also can't provide help to the B cells in forming antibodies or long-term immunity.
"It was thought that if you don't have SAP, you don't get positive signaling from these receptors," she says. "But we found that in the absence of SAP, it's not that these receptors don't signal, but rather, they recruit a strong negative signaling molecule, an enzyme, which actively turns off signaling." When the researchers blocked one of the receptors or the enzyme, they found that they could halt the negative signal and, in fact, allow the T cells to kill EBV-infected B cells.
Most patients with XLP have few good treatment options, beyond perhaps bone marrow transplant. While new therapies are years away, Dr. Schwartzberg is hopeful that her team's ongoing research efforts, including its most recent findings, will continue to "provide new insights to the mechanism of the disease, and open up new possibilities for therapeutic approaches."
Dr. Schwartzberg notes that in many chronic infections, such as HIV and hepatitis C virus, T cells can be characterized by a condition called "exhaustion," where they don't work properly.
"These findings raise the question of whether these same types of negative signals we see in XLP are involved in exhaustion," she says, adding these results "provide insight not only into how to approach this disease, but also how to approach many long-term viral infections where we don't have good treatments."
