Last updated: March 13, 2018
Frequently Asked Questions about IGNITE II
Frequently Asked Questions about IGNITE II
- RFA-HG-17-008: Implementing Genomics in Practice (IGNITE) II: Pragmatic Clinical Trials - Clinical Groups (U01)
- RFA-HG-17-009: Implementing Genomics in Practice (IGNITE) II: Pragmatic Clinical Trials -Enhanced Diversity Clinical Groups (U01)
- RFA-HG-17-010: Implementing Genomics in Practice (IGNITE) II: Pragmatic Clinical Trials -Coordinating Center (U01)
Can you describe the nature of a pragmatic clinical trial in more detail?
Prospective trials evaluating the validity and utility of genomic medicine interventions are needed for the implementation of genomics into clinical practice. Pragmatic clinical trials exist on a continuum with randomized clinical trials, where traditional randomized clinical trials are often designed to demonstrate efficacy under ideal conditions. Pragmatic clinical trials, however, are designed to assess effectiveness in routine settings. Pragmatic clinical trials are therefore better integrated with routine clinical care and are designed to produce practical evidence that can ultimately be used to improve practice and policy. IGNITE II applications should propose trials that focus on routine, "real world" settings that will ultimately be useful in assessing the impact of incorporating a genomic medicine intervention into clinical practice and if successful could be rapidly implemented in most clinical care settings.
Further discussion of pragmatic clinical trials can be found at Ford I, Pragmatic Trials.
Could previously enrolled patients with retrospectively collected data be included in the minimum sample size to be enrolled by a Clinical Group (CG)?
Each CG is expected to consent and enroll a minimum of 3,000 patients across all IGNITE II implemented protocols, and to complete screening and enrollment within a 12-month period of initiation of each protocol. While you can include patients previously enrolled in another clinical study, data for the pragmatic clinical trials should be collected prospectively.
How many diseases, patient groups, and geographic regions should my application include?
IGNITE II will assess approaches for real-world application of genomic medicine in diverse clinical settings. As stated, applicants to RFA-HG-17-008 are expected to recruit a minimum of
35% of patients who come from racial or ethnic minority populations, underserved populations, or populations who experience poorer medical outcomes. Applicants to the companion RFA HG-17-009 are expected to recruit a minimum of 75% of such patients. Applicants to both RFAs are expected to recruit 50% of patients from diverse clinical settings such as community hospitals, family medicine clinics, and primary care practices.
Beyond the minimum requirements, the strongest applications will have access to a broad range of diseases, patient groups, expertise, and geographic regions not only relating to the pragmatic trial proposed in their application, but also those that would give the greatest likelihood of participating in PCTs proposed by other groups for network-wide implementation. While this may be challenging as the PCTs proposed by other groups will be unknown at the time of application, demonstrating the ability to participate in a wide a range of PCTs will significantly strengthen an application.
Will cancer-related applications be considered for funding?
Given the extensive ongoing work in implementing genomics in cancer treatment, cancer-only trials will be considered non-responsive to the IGNITE II RFAs and will be returned to the applicant. Trials evaluating germline cancer susceptibility as one component in a broader analysis of susceptibility to a variety of diseases may be acceptable. However, applications in which cancer susceptibility is a major component of the proposed analysis will be considered a low priority for NHGRI funding. Applicants are encouraged to contact NHGRI to discuss their proposed trial and patient population before formally submitting their applications.
Are costs for genomic testing allowable?
Yes, genomic testing in a CLIA-compliant manner can be included in the proposed budget. Applicants should be aware of the instructions in Section IV., Application and Submission Information, "R&R or Modular Budget" and "R&R Subaward Budget" that provide guidance on information to be included in the application budget.
What is the scope of responsibility of the coordinating center?
Given that IGNITE II will rely on network adoption of multiple pragmatic clinical trials, the coordinating center will be integral to IGNITE II's successful evaluation of genomic medicine interventions. In brief, the coordinating center will lead, in collaboration with the Clinical Groups, all aspects of network-wide protocol adaptation, execution, and analyses, development and statistical modeling, and final analysis of primary and secondary network-wide clinical trial outcomes in collaboration with the Clinical Groups network-wide. For a full description of the IGNITE II Coordinating Center responsibilities, please refer to the Research Approach and Research Strategy sections of RFA-HG-17-010.
What specific information should the letters of support for each clinical site include?
Letters of support from the Clinical Sites should document commitment from site leadership to participate fully in IGNITE II and are encouraged to demonstrate appropriate commitment and resources such as protected time, facilities, space, or resources for the Clinical Site; describe their experience in enrolling patients in clinical research studies; and provide a summary of the site's research experience and capabilities similar to the format below.

Printable document containing Site Information form ![]()
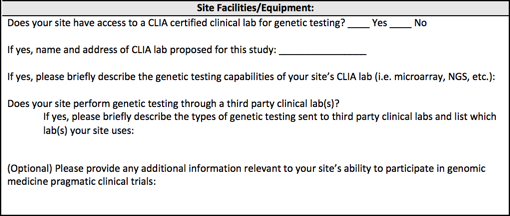
Printable document containing the Site Facilities/Equipment form ![]()
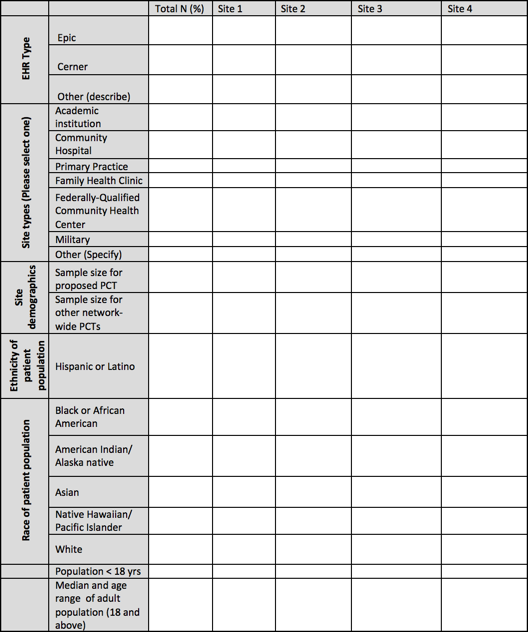
How should the clinical settings and characteristics of clinical sites within an applicant's Clinical Group be described?
Applicants should provide a summary table describing the Clinical Sites comprising their Clinical Group that lists the clinical setting (e.g. academic institution, community hospital, primary care practice, etc.) and the site's characteristics. A table similar to the sample below may be useful for organizing and providing this information.
Why is the ethical, legal and social implications (ELSI) research component being funded separately through a supplement?
To ensure that the network-wide ELSI research study is relevant to the pragmatic clinical trials selected for implementation, the ELSI research study will be developed after the PCTs are prioritized and selected to go forward. The ELSI research study will be collaboratively developed by the CGs after award based on the most significant ELSI issues in the selected PCTs and the strengths of the IGNITE II Network. The ELSI research study should not be proposed in the current application. However, applicants should describe unique strengths of the proposed research team, including experience conducting ELSI research.
How will the pragmatic clinical trials to be implemented network-wide be selected?
Network-wide pragmatic clinical trial protocols proposed by applicants will be prioritized after award by a Protocol Review Committee (PRC), which will include experts in genomic medicine, clinical trial design, biostatistics, outcome measures, and other areas of expertise as needed, and relevant stakeholders. The PRC will provide a written critique of each proposal and a final prioritization to the NHGRI. Once protocols are selected to move forward, the Steering Committee, comprising the Principal Investigators of each group, the CC PI, and NHGRI personnel, will then adapt prioritized protocols for expansion across the IGNITE II Network.
Does my pragmatic clinical trial need to involve randomization (of patients and/or clinical sites)?
Randomization of patients and/or clinical sites to a control or intervention arm(s) is expected. Investigators considering proposing a non-randomized trial must provide a strong justification during their pre-application discussions with NHGRI staff.
What is considered a specialized medical center?
For the purposes of these RFAs, by specialized medical centers we mean centers that have extensive experience providing genetic or genomic services or receive referrals for genomic healthcare services.
Would a pediatric clinical trial be responsive to the RFA?
Yes, as long as it meets the other criteria of the RFA for generalizability, clinical importance, feasibility, potential impact, and cost.
Would a site proposing a pediatric clinical trial also have to demonstrate its ability to recruit adults for other trials to be conducted by the IGNITE II network, and vice versa (a site proposing an adult clinical trial demonstrating its ability to recruit children)?
The RFA encourages a focus on projects that can be applied in a wide range of clinical settings and across a wide range of clinical sites. While studies limited to a specific age group can certainly meet this and the other RFA criteria for high-priority trials, it will be important for a Clinical Group to demonstrate it has the capacity to implement trials across a wide age range. For budgeting purposes, however, Clinical Groups should provide an estimate that includes the Clinical Sites that can conduct the trial it is proposing. If those sites are constrained by age or other characteristics of the proposed trial population, Clinical Groups should also identify and describe other Clinical Sites that are part of their Clinical Group and would be available to participate in other network-wide trials ultimately selected for IGNITE II.
Can you clarify the budget figure of $5.1M direct costs per clinical trial (Section IV.2, "R&R or Modular Budget")?
In year 1, application budgets are limited to $250,000 direct costs (DCs) for base level personnel and infrastructure support. For year 2-5, the budget is limited to $760,000 DCs per year to support base level personnel and infrastructure plus the Clinical Group (CG)'s share of the proposed pragmatic clinical trial. The assumptions are that 4-6 CGs will be awarded and 2-4 pragmatic clinical trials (PCTs) will be chosen for implementation. Please note, when considering your proposed PCT that the trial should have a maximum estimated direct cost across all IGNITE CGs of no more than $5.1M (in addition to base CG and CC support) over years 2-5. Specifically, $760,000 DCs - $250,000 DCs = $510,000 DCs (one CG's share of the proposed pragmatic trial) x 5 (4-6 CGs) =$2,550,000 DCs x 4 years = $10,200,000 DCs. Assuming that at least 2 PTCs would be implemented in IGNITE II, then, the maximum amount a PTC should cost over 4 years is $10,200,000 / 2 PTCs = $5,100,000 DCs."
Because applicants can not predict what trials will be implemented, we are asking applicants to use the following structure for budgeting purposes: up to $250,000 direct costs in year 1, $760,000 in year 2, $760,000 in year 3, $250,000 in year 4, and $250,000 in year 5. This will include the budget for your share (approximately 1/5) of your proposed PCT over years 2 and 3, with the understanding that additional funds might be provided as a supplement in the following years for other CG's potential PCTs to be implemented network wide. The specifics of the additional funds will be determined once the network is established and the PCTs to be implemented are decided on.
Can one Clinical Site be proposed to be part of more than one Clinical Group application?
Yes, a Clinical Site can be included in multiple Clinical Group applications. Support letters from such Clinical Sites to each application they are participating in should clearly describe how possible overlaps and conflicting responsibilities will be identified and managed if more than one application is awarded.
Will the research proposed in the Clinical Group and Enhanced Diversity Clinical Groups RFAs be considered "delayed onset human subjects research"?
The NIH recognizes that certain research applications may be submitted to NIH with the knowledge that human subjects will be involved during the period of support, but definite plans for this involvement cannot be described in the application. This situation is described as "delayed onset human subjects research" and the NIH has established policy related to such research. This policy describes the major categories of research that could be considered delayed onset and the requirement for NIH prior approval before initiating human subjects research. Please note, it is up to the applicant institution to make the determination as to whether the research proposed in IGNITE II applications should be considered delayed onset human subjects research
The RFA states that an independent, network-wide, Data and Safety Monitoring Board will be established by NHGRI. Should an application include a DSMB description as it relates to that application's proposed PCT?
Applicants should provide a description of a DSMB structure for the particular clinical trial being proposed, but should not name or contact any potential DSMB member. The application can acknowledge that NHGRI will oversee the establishment of the DSMB if the proposed trial is implemented, but at the same time can include what an appropriate DSMB structure would look like. This way, a reviewer who expects to see a DSMB plan by default wouldn't look unfavorably on the lack of any description at all.
IGNITE II Webinar Information
Date: Thursday, August 17th, 2017
Time: 1:00 - 3:00 PM EDT
Dial-in (toll number): 1-650-479-3208
The intent of the pre-application webinar is to provide an overview of the IGNITE II FOAs and to address questions pertinent to preparing applications. The webinar is optional and not required for application submission.
Prospective applicants with inquiries concerning the FOAs who are unable to participate in the Webinar are encouraged to view the summary of questions and answers after the Webinar. For any additional questions, contact the Scientific/Research contacts listed in RFA-HG-17-008, RFA-HG-17-009, and RFA-HG-17-010.
Slides from the IGNITE II Webinar 
Agenda:
1:00 p.m. Overview of IGNITE II CG and EDCG RFAs
1:15 p.m. Overview of IGNITE II CC RFA
1:20 p.m. Application Budget
1:30 p.m. Questions and Answers
2:00 p.m. FAQs
2:10 p.m. Overview of Scientific Review
2:20 pm Questions and Answers Continued
3:00 p.m. Webinar adjourned*
*Please note that the webinar will adjourn early if all questions are answered before 3:00 p.m.
