Last updated: August 01, 2014
Improving The Detection Of Heart Transplant Rejection With Dna Sequencing
Genome Advance of the Month
Improving the detection of heart transplant rejection with DNA sequencing
By Elizabeth Burke, Ph.D.
Intramural Postdoctoral Fellow, NHGRI
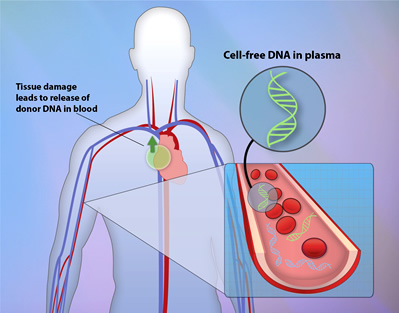
With the hope of reducing the risks associated with heart transplants, scientists have been working diligently to develop a new, less invasive method to test for rejection. Rejection, which occurs when a patient's immune system attacks and destroys the new organ as if it were a harmful virus or bacteria, is currently detected by cutting off a small piece of the transplanted heart and examining it for cell death (endomyocardial biopsy). However, a group of researchers at Stanford University have recently developed a new test that prevents the need for this surgery. They discovered that dying cells within the rejected heart release small pieces of DNA, called cell-free, donor-derived DNA (cfdDNA), into the bloodstream that can be detected through a simple blood draw.
June's Genome Advance of the Month features this group's work, published in the June 18, 2014, issue of Science Translational Medicine, which demonstrates how sequencing and quantification of cfdDNA can be used to effectively determine whether or not a heart transplant will be rejected.
The researchers examined the efficacy of cfdDNA monitoring in a total of 21 pediatric and 44 adult patients who received heart transplants. To do this, they drew blood samples from the 65 patients and their heart donors prior to the transplant and sequenced their cell-free circulating DNA. The sequences were used to screen for single nucleotide polymorphisms (SNPs), which are common single nucleotide (A, T, C or G) variations in DNA that can differ from person to person. Thousands of SNPs that differed between each donor/recipient pair were identified in order to generate unique DNA profiles, or genotypes, that were used to distinguish donor DNA from recipient DNA.
After the heart transplant, the recipients' blood was sampled routinely to measure the amount of the circulating DNA that came from the donor (cfdDNA) as well as from the recipient. If abnormally high levels of cfdDNA were detected, this indicated that the recipient's immune system was beginning to attack the new heart.
The team of researchers first determined what amount of cfdDNA is normal following a transplant by looking at cfdDNA levels in patients that did not experience a rejection event. In these patients, the researchers found that the cfdDNA levels were elevated (up to six percent of total circulating DNA) on the first day after transplant, but were reduced after one week to a low baseline level (less than two percent of total circulating DNA) where it remained throughout the first year. In contrast, patients that did experience a rejection event from one week to one year after their transplant were found to have increased levels that ranged from two percent to greater than ten percent depending on the severity of their reaction.
To establish the accuracy of this method, they directly compared cfdDNA monitoring to endomyocardial biopsies in diagnosing heart rejection by performing both methods on each patient. They found that levels of cfdDNA were significantly lower in patients determined to be rejection-free (by biopsy) compared to those diagnosed with mild rejection. Furthermore, cfdDNA levels increased as the severity of the rejection event (mild, moderate and severe) increased. These findings demonstrated that cfdDNA measurement is a quantitative approach that can determine the severity of a rejection event as accurately as a biopsy.
However, the level of accuracy for cfdDNA monitoring relative to endomyocardial biopsy was found to be dependent when cfdDNA was measured, in that its performance improved up until four months after the transplant. The accuracy was also found to decrease as the patients' age progressed. This variability is most likely caused by age-related differences in immune responses, as the immune system's function is known to decrease with age.
Although this method's performance is slightly altered with time and age, its superior ability to detect rejection compensates for these minor drawbacks. The scientists found that the cfdDNA levels were significantly elevated for up to five months before rejection could be detected by endomyocardial biopsy. Thus, the use of cfdDNA monitoring has the potential to identify a rejection event several months earlier than what is currently possible.
Overall, the authors of the study demonstrated that cfdDNA screening is an effective, less invasive method for transplant monitoring. By providing an alternative to endomyocardial biopsy, this method reduces the risks, patient discomfort and expense that are currently associated with heart transplant rejection monitoring. Moreover, their data suggests that it will substantially improve the chances of survival and good health for heart transplant recipients by allowing for earlier diagnosis and treatment that may prevent the rejection event from occurring.
With such remarkable results, the next and most important question is: When will this become available for everyday use in the clinic? Dr. Stephen Quake, one of the senior authors of the article, remarked, "This study shows that cfdDNA is ready for prime time use and I expect it will very quickly transition into the clinic, perhaps as early as 2015." This is indeed exciting news for all of those out there who are awaiting a new heart.