Last updated: May 19, 2012
Nhgri Fy 2010 Congressional Justification
Archived Page
This page has been archived and is provided for historical reference purposes only. The content and links are no longer maintained and may now be outdated.NHGRI FY 2010 Justification of Estimates for Congressional Appropriations Committee
- Organization Chart
- Appropriation Language
- Amounts Available for Obligation
- Budget Mechanism Table
- Budget Authority by Activity
- Major Changes in Budget Request
- Summary of Changes
- Budget Graphs
- Justification Narrative
- Budget Authority by Object
- Salaries and Expenses
- Authrorizing Legislation
- Appropriations History
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
- New Positions Requested
To be used as a printable hard copy.
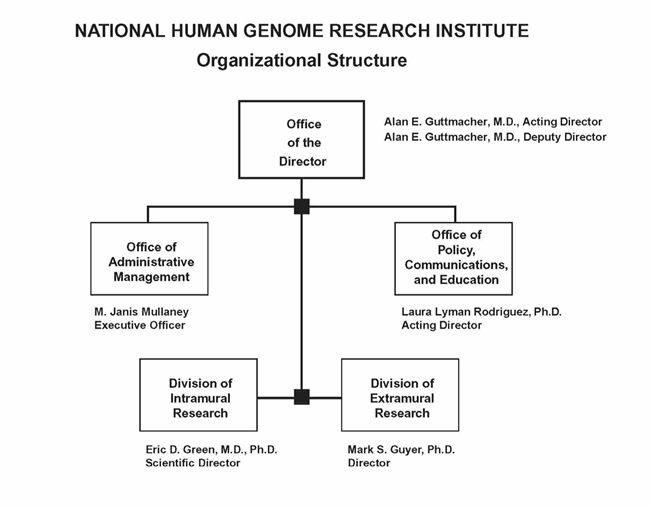
Organization Chart
Appropriation Language
For carrying out section 301 and title IV of the Public Health Services Act with respect to human genome research, $502,367,000 $509,594,000 (Department of Health and Human Services Appropriation Act, 2009)
Amounts Available for Obligation 1
| Sources of Funding | FY 2008 Actual | FY 2009 Estimate | FY 2010 Estimate |
|---|---|---|---|
| Appropriation | $495,434,000 | $502,367,000 | $509,594,000 |
| Type 1 Diabetes | 0 | 0 | 0 |
| Rescission | -8,655,000 | 0 | 0 |
| Supplemental | 2,589,000 | 0 | 0 |
| Subtotal, adjusted appropriation | 489,698,000 | 502,367,000 | 509,594,000 |
| Real transfer under Director's one-percent transfer authority (GEI) | 16,035,000 | 0 | 0 |
| Comparative transfer under Director's one-percent transfer authroity (GEI) | -16,035,000 | 0 | 0 |
| Subtotal, adjusted budget authority | 489,368,000 | 502,367,000 | 509,594,000 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | 489,368,000 | 502,367,000 | 509,594,000 |
| Unobligated balance lapsing | -23,000 | 0 | 0 |
| Total obligations | 489,345,000 | 502,367,000 | 509,594,000 |
1 Excludes the following amounts for reimbursable activities carried out by this account:
FY 2008 — $22,884,000 FY 2009 Estimate — $52,290,000 FY 2010 Estimate — $53,022,000.
Excludes $111,000 Actual in FY 2008; Estimate $247,000 in FY 2009; and Estimate $133,000 in FY 2010 for royalties.
Budget Mechanism Table
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research.
FTTP = Full-time temporary equivalent
FTE = Full-time equivalent
Budget Authority by Activity
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
Major Changes in Fiscal Year 2010 Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2010 budget request for NHGRI, which is $7.227 million more than the FY 2009 Estimate, for a total of $509.594 million.
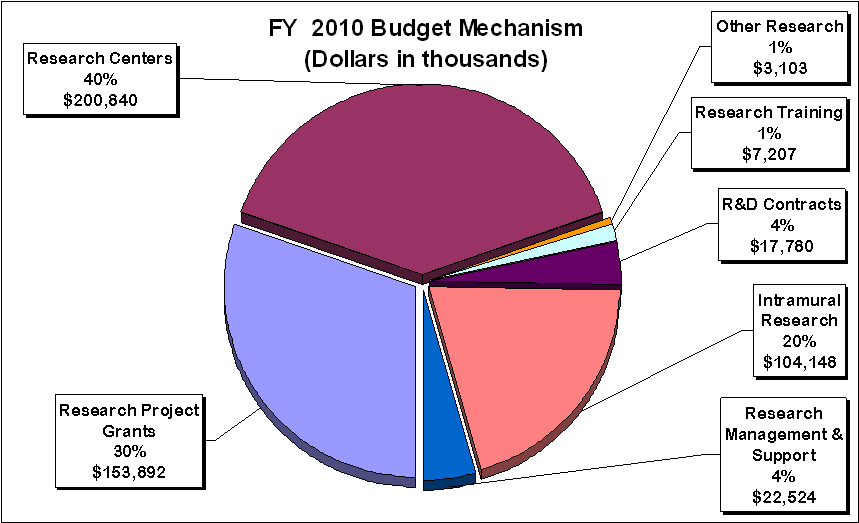
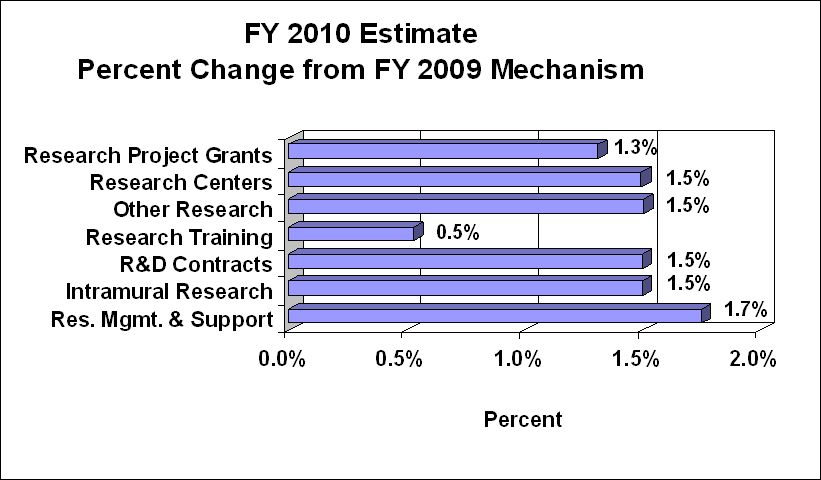
Research Project Grants (RPGs) (+$1.991 million, total $153.892 million): The NIH Budget policy for RPGs in FY 2010 provides a two percent inflationary increase in noncompeting awards and a two percent increase in the average cost for competing RPGs. NHGRI will support a total of 271 Research Project Grant (RPG) awards in FY 2010. Noncompeting awards will increase by nine awards and by $1.306 million. Competing RPGs will increase by two awards and $1.800 million. NHGRI will continue to support new investigators and to maintain an adequate number of competing RPGs.
Research Centers (+$2.962 million; total $200.840 million): The FY 2010 budget policy provides a two percent average cost increase. The number of research centers is expected to continue at 45 in FY 2010.
Medical Sequencing (+$5.000 million; total $64.766 million): Large-scale sequencing technology has improved significantly. The additional $5.000 million provided will increase the proportion of the Institute's sequencing program to support new opportunities to apply genomic tools to the study of human disease.
Large-scale Sequencing (Non-Medical) (-$10.000 million; total $25.000 million): Emphasis within the NHGRI Large-scale Sequencing Program is continuing to shift in FY 2010 from non-medical to medical sequencing and to sequencing for The Cancer Genome Atlas. The $10.000 million decrease in the Non-Medical component of the program is due to this shift in the distribution of sequencing activities and is made possible by improvements in process efficiency. The Non-Medical component of the program will maintain, or possibly increase, the total amount of data it can generate within the reduced funding, because of expected increased output and decreased costs afforded by next-generation sequencing technologies.
The Cancer Genome Atlas (+$5.450 million, total $22.500 million): The program is increasing because large-scale sequencing technology has improved significantly and this additional funding will support new opportunities to use this technology to study cancer.
Translational Genomics (+$0.909 million; total $25.516 million): Expanded efforts in this program in FY 2010 will focus on genome-wide association studies (GWAS) for several additional diseases (to be chosen through a peer-reviewed competition), and drug and treatment response. New computational and experimental methods to follow up on the GWAS results to determine the specific genetic variations responsible for the diseases will be developed as well.
Summary of Changes
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
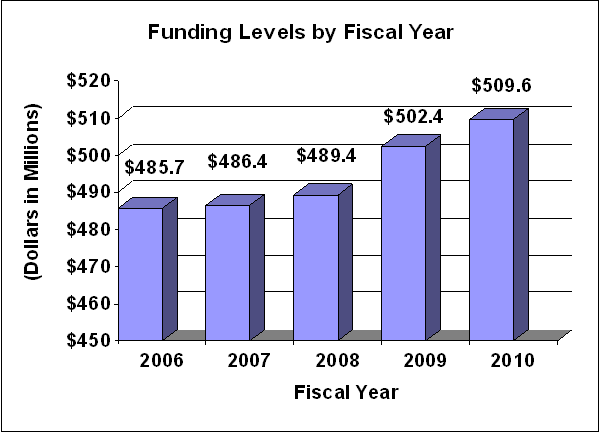
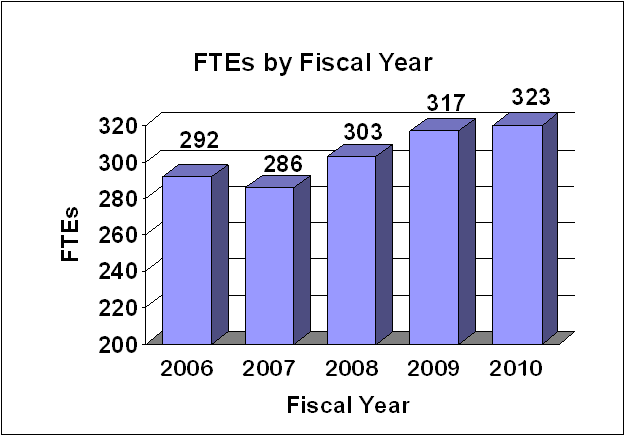
Fiscal Year 2010 Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism:
Changes by Selected Mechanism:
Justification Narrative
Authorizing Legislation:
Section 301 and title IV of the Public Health Service Act, as amended.
Budget Authority:
|
This document provides justification for the Fiscal Year (FY) 2010 activities of the National Human Genome Research Institute (NHGRI), including HIV/AIDS activities. Details of the FY 2010 HIV/AIDS activities are in the "Office of AIDS Research (OAR)" Section of the Overview. Details on the Common Fund are located in the Overview, Volume One. Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
In FY 2009, a total of $127,035,000 American Recovery and Reinvestment Act (ARRA) funds were transferred from the Office of the Director. These funds will be used to support scientific research opportunities that help support the goals of the ARRA. The ARRA allows NIH to execute these funds via any NIH funding mechanism. Funds are available until September 30, 2010. These funds are not included in the FY 2009 Omnibus amounts reflected in this document.
DIRECTOR'S OVERVIEW
After leading the Human Genome Project to the successful completion, in 2003, of its extraordinary goal of sequencing the entire human genome, NHGRI expanded its mission to encompass a broad range of studies aimed at understanding the structure and function of the human genome and its role in health and disease. To that end, the NHGRI supports cutting edge genomics and translational research with an emphasis on the development of resources and technology that will accelerate research and the application of new tools and findings to human health and disease. The ultimate aim of the NHGRI scientific and ethical research portfolios is to enable truly preemptive, predictive, personalized, and participatory health care.
Windfall of Discoveries of the Genetic Basis of Disease
The Nation's previous investments in the HGP and the International HapMap Project have moved us closer to a future that uses genomic information to diagnose, treat, and prevent disease. With unprecedented speed, researchers have used HapMap-enabled genome-wide association studies (GWAS) to identify a stunning number — over 130 in 2008 alone — of genetic factors associated with major causes of morbidity and mortality in the United States, such as diabetes, cardiovascular disease, lung and prostate cancer, and inflammatory bowel disease. Three recent GWAS of lung cancer have implicated genes related to nicotine addiction in disease causation, underscoring the possibility of genetic interactions with one of the strongest known environmental risk factors for lung cancer - cigarette smoking. Identification of gene variants associated with disease raises the possibility of using genetic testing, in combination with family history information, to identify susceptible, pre-symptomatic subjects for screening and preventive therapies.
Technology Development
Fueling the swift pace of genomic discoveries is NHGRI's continuing commitment to the development of innovative sequencing technologies, which reduces the cost and increase the speed of DNA sequencing. In the past decade, sequencing costs have decreased more than 50-fold, in part because the HGP delivered – beyond the complete DNA sequence – a wealth of tools, technologies, and process improvements. In FY 2008, the Institute supported investigators to develop near-term technologies to sequence a mammalian-sized genome for $100,000 and longer-term, revolutionary technologies to sequence a mammalian-sized genome for $1,000 or less. Excellent progress is expected toward achieving both goals by the year 2014. Importantly, the $1,000 Genome would enable health care professionals to personalize prediction, diagnosis, treatment, and preemption of disease to each person's unique genetic profile by bringing DNA sequencing into a cost-effective range for clinical use.
Cancer Genomics
The Cancer Genome Atlas (TCGA), initiated in FY 2007, applies a comprehensive, large-scale genomic analysis approach to cancer research. It is jointly supported and led by the NHGRI and the National Cancer Institute (NCI) and completed its pilot phase in FY 2009. In FY 2008, the first results of this project were obtained for the most common form of brain cancer, glioblastoma multiforme (GBM). Among other important findings, the research identified a potential mechanism of resistance to a common chemotherapy drug used for brain cancer. These first results from the TCGA pilot project represent an exciting new discovery and provide proof of concept about the value of thismulti-dimensional analysis of the molecular characteristics of cancer in humans. The NHGRI will continue to support, independently, additional cancer research through the Tumor Sequencing Project, which will support comparable analysis of large-scale identification of genomic changes in different tumor types.
The Undiagnosed Diseases Program (UDP)
An important clinical program, launched in 2008, the UDP aims to provide answers to patients with mysterious conditions that have eluded diagnosis, through the conduct of an intensive review and analysis by a multi-disciplinary team of experts. The UDP is jointly led by the NHGRI, the NIH Clinical Center, and the Office of Rare Diseases Research and focuses on the most puzzling medical cases referred to the NIH by physicians across the nation.
The NIH Therapeutics for Rare and Neglected Diseases (TRND) Program
The TRND, launched in FY 2009, is focused on leveraging technologies and knowledge learned in the NIH Chemical Genomics Center to improve the Nation's ability to identify effective therapeutics for the diseases that affect humans. In general, developing effective therapeutics is a time-consuming, expensive, multi-step process with high likelihood of failure. The private sector has focused on those diseases for which success promises a high return on investment. It typically ignores the more than 6,000 diseases that are either "rare" (occurring in fewer than 200,000 Americans) or "neglected" (occurring predominantly in individuals, usually in the developing world, with little ability to pay for medications). The TRND aims to use high throughput and multi-disciplinary approaches in drug development for rare and neglected diseases in a way that builds new paradigms that can be generalized to other research and production settings. A focus on this part of the drug development pathway should reduce the time and expense needed to traverse the remaining steps and significantly increase the private sector's interest in producing new therapeutics for rare and neglected diseases.Moving toward Personalized Genomic Medicine: An Innovative and Proactive Approach
Two clinical genomics initiatives launched in FY 2007 are now in full stride. The first, ClinSeq, is a pilot study aimed at developing the technologic and procedural infrastructure to facilitate large-scale medical sequencing in a clinical research setting. The second, the Multiplex Initiative, is a research study intended to provide genetic susceptibility testing for several common health conditions, such as cardiovascular disease and osteoporosis, and to evaluate patients' reactions to the testing and receipt of results.
Full realization of the potential of genomic medicine requires a multi-pronged approach that includes not only basic science advances, but also health applications research, the education of health professionals and the public, and community involvement in the analysis and interpretation of the complex ethical, legal, and social issues raised by this powerful new level of knowledge about each of us as individuals.
Overall Budget Policy: The NHGRI will continue to support new investigators and to maintain an adequate number of competing RPGs. In order to maximize the number of competing RPGs that can be made, NHGRI's review and approval funding process will follow the NIH budget policy in providing 2.0 percent inflationary increases for non-competing and competing grants. Investigator-initiated research projects and new investigator research and career development are among the Institute's highest priorities. The NHGRI carefully evaluates investigator-initiated requests to submit grant applications for all large programs. The Institute maintains a balance between solicitations issued to the extramural community in areas that need stimulation and funding made available to support investigator-initiated projects. Intramural Research and Research Management and Support receive an increase in FY 2010 to help cover the cost of pay and other increases.
FY 2010 JUSTIFICATION BY ACTIVITY DETAIL
Program Descriptions and Accomplishments
EXTRAMURAL RESEARCH
Basic Genomics
Large-scale Sequencing
One of the primary objectives of contemporary biomedical research is to define and understand how the human genome functions, how misfunction leads to disease, and how that knowledge can be used to develop new preventative strategy, diagnostic methods, and therapies. Comparison of the genome sequence of humans with that of other organisms identifies regions of similarity and difference, providing insight into the evolution, structure, and function of human genes and pointing to new strategies to combat human disease. Therefore, genome sequencing of multiple non-human species as a window into the human genome remains an important approach to biomedical research and a priority for the NHGRI.
Currently, 197 genomes are either in the pipeline or have been completed by the NHGRI. In FY 2008, one of the most interesting genomic sequences completed was that of the duck-billed platypus, which provided new clues on how fundamental mammalian biological processes have evolved. Ongoing sequencing targets include several more non-human primates, additional mammals, fungi, and multiple strains of yeast. The NHGRI funds this work by supporting three large-scale sequencing centers that are world leaders and renowned for their cost effective and high quality work.
Budget Policy: The FY 2010 budget estimate for large-scale sequencing (non-medical) is $25.000 million, a decrease of $10.000 million or 28.6 percent from the FY 2009 Estimate. This decrease represents a continuation of on-going reprioritization and is balanced by increases in the Medical Sequencing and The Cancer Genome Atlas large-scale sequencing components (see below), so that the total amount of NHGRI spending on large-scale sequencing will remain constant. FY 2010 will be the year in which the on-going major transition in sequencing technology will be fully implemented. The capillary gel electrophoresis-based sequencing instruments previously used to sequence the human genome will be supplanted with a mix of sequencing approaches dominated by the new, next-generation single molecule-based sequencing instruments (that were developed with a significant amount of support from the NHGRI Sequencing Technology Program, a prime example of public-private partnership and economic development directly attributable to NIH funding). One effect of implementation of the "'next-gen" technologies will be a significant increase in the overall capacity of the NHGRI Sequencing Program. This will allow the Institute to increase the amount of sequence data generated in the Large-Scale Sequencing (Non-Medical) component while decreasing the amount of funding dedicated to that area. At the same time, the Institute will be able to devote a larger proportion of the overall large-scale sequencing effort in FY 2010 to projects directed toward understanding disease, specifically, Medical Sequencing (see below) and The Cancer Genome Atlas (see below). The activity in the Large-Scale Sequencing (Non-Medical) component will continue the NHGRI's signature efforts to generate the genomic sequence data from many sources that are needed to reveal the functional components of the human genome.
Medical Sequencing
As more is learned about the genomic contribution to disease, sequence information will become ever more important for providing medically relevant information to individuals. When it becomes affordable to sequence any individual's genome completely, such information will allow estimates of future disease risk and improve the prevention, diagnosis, and treatment of disease. The NHGRI's medical sequencing program, initiated in FY 2007, aims both to drive continued technology improvement (which lowers the cost of genome sequencing) and to produce data useful to biomedical research.Seven studies are currently underway to identify the genes responsible for several relatively rare, "single-gene" diseases and to survey the range of gene variants that contribute to certain common diseases. In FY 2008, a number of additional medical sequencing projects were initiated:
- Sequencing the genomic regions identified in genome-wide association studies as containing genetic components underlying common diseases, such as diabetes, breast cancer, schizophrenia or Crohn's disease;
- Sequencing the genomes of important human pathogens, such as those that cause malaria and sleeping sickness, and their invertebrate vectors (in collaboration with the National Institute of Allergy and Infectious Disease (NIAID) ); and
- The Cancer Genome Atlas project (see below). The NHGRI's Medical Sequencing Working Group continues to chart a course toward clinical application of medical sequencing, as well as to provide guidance on setting policies for ethical, legal, and social issues arising from the program.
Budget Policy: The FY 2010 budget estimate for medical sequencing is $64.766 million, an increase of $5.000 million or 8.4 percent over the FY 2009 Estimate. Medical sequencing continues to be an area of growth for the NHGRI. With the introduction of next-generation sequencing instruments on a production scale, many new opportunities have been created to apply genomic tools to the study of human disease and the application of that information to the development of new approaches to disease management. In FY 2010, the NHGRI will continue the increase of funds that the Institute spends on the Medical Sequencing component of its Large-Scale Sequencing program.
The Cancer Genome Atlas
The Cancer Genome Atlas (TCGA) is a pilot project, jointly supported and led by the NHGRI and the National Cancer Institute (NCI), which was initiated in FY 2007 to apply a comprehensive, large-scale genomic analysis approach to cancer research. TCGA is designed to develop and test the complex scientific and technological frameworks needed to identify the mutations and other complex genomic changes associated with each type of cancer. Three NHGRI-supported sequencing centers provide the genomic sequencing capability for TCGA. In FY 2008, the first results of this genomic approach were obtained for the most common form of brain cancer, glioblastoma multiforme (GBM). The research identified many of the gene mutations involved in GBM, including mutations in three genes that were previously unrecognized to occur with significant frequency in GBM; and it delineated core pathways that are disrupted in this type of brain cancer. Another very exciting result was an unexpected observation that points to a potential mechanism of resistance to a common chemotherapy drug used for brain cancer. These first results from the TCGA pilot project represent an exciting indication of the value of the multi-dimensional analysis of the molecular characteristics in human cancer.In the next one to two years, the focus of TCGA will be on two other common cancers, squamous cell lung cancer and ovarian cancer, as well as further analysis of glioblastoma (brain cancer).Another component of the NHGRI Cancer Sequencing Program is a collaborative effort among the NHGRI large-scale sequencing centers and cancer biology experts studying lung adenocarcinoma. In FY 2007, this group used comprehensive genomic analysis to reveal previously unknown genes that play an important role in that form of cancer, and on-going efforts have continued to reveal more information about the genomics of lung cancer.
Budget Policy: The FY 2010 budget estimate for TCGA is $22.500 million, an increase of $5.450 million or 32.0 percent over the FY 2009 Enacted. In its pilot phase, the TCGA has met many of its original objectives, most importantly the demonstration of the feasibility and value of a genomic analysis of the genomes of specific tumor types.
Genomic Function
The NHGRI supports research to identify and characterize the function of all parts of our genome and to understand their biological relevance. Efforts to uncover functional elements are not limited to the human genome, since understanding the genomes of other, "model," organisms can also give insight into the structure and function of the human genome.
Launched in FY 2003 as a pilot, the ENCyclopedia of DNA Elements (ENCODE) Project successfully demonstrated its ability to provide important information about the structure and function of 1 percent of the human genome. In FY 2007, the NHGRI implemented a full-scale ENCODE Project to examine the entire human genome for sequence-based functional elements, and initiated modENCODE, which has similar goals for the analysis of the genomes of two important model organisms. The NHGRI has supported two trans-NIH initiatives to develop publicly available resources critical to accelerating research on genomic function: 1) the Mammalian Gene Collection (MGC), which successfully obtained full-length clones for every human and mouse gene, completed in FY 2008; and 2) the Knockout Mouse Project (KOMP), a collaboration with European and Canadian groups that seeks to produce a mutation in every mouse gene.
Budget Policy: The FY 2010 budget estimate for Genomic Function programs is $61.602 million, an increase of $1.208 million or 2.0 percent from the FY 2009 Estimate. Activity in Genomic Function will remain essentially constant, maintaining the proportion of the NHGRI extramural budget devoted to this area. It should be noted, however, that this is another area in which the introduction of next-generation sequencing technology will enable a significant increase in the amount of data generated without an increase in cost. The Institute also will continue to fund meritorious investigator-initiated applications submitted in response to announcements that encourage new technologies and new approaches to the analysis of genomic function.
Genomic Variation
Although the genome sequence variation between two people is less than one percent, this tiny difference underlies a variety of observable characteristics ranging from the benign, such as hair or eye color, to disease, such as diabetes, cancer, Alzheimer's, or heart disease. The NHGRI-led International HapMap Project charted the common patterns of genetic variation in the world's population by identifying and cataloging single letter spelling variations in our genome's alphabet, referred to as single nucleotide polymorphisms, or SNPs. The goal of the HapMap is to provide the resource necessary to identify disease-causing variants with the potential of using these discoveries to develop treatments.
In FY 2007, the promise of the HapMap as an unparalleled resource for human genetics was dramatically realized, with the identification of a host of new genes involved in a number of common diseases. Additionally, the second generation haplotype map, Phase II HapMap, was completed and published in FY 2007. At three times the resolution of the original, Phase II has facilitated many new studies to investigate the link between genetic variation and other factors involved in health and disease, including susceptibility to infection, response to environmental factors, and drug efficacy. In FY 2008, the NHGRI initiated the 1000 Genomes Project to obtain an even more complete catalogue of human sequence variation by using new and more cost-effective sequencing technologies to sequence the genomes of approximately 1000 individuals.
Budget Policy: The FY 2010 budget estimate for Genomic Variation programs is $14.669 million, an increase of $287 thousand or 2.0 percent over the FY 2009 Estimate. Activity in Genomic Variation will remain essentially constant, maintaining the proportion of the NHGRI extramural budget devoted to this area. However, emphasis within this program will shift to the analysis of the data generated in the 1000 Genomes Project. The NHGRI will also continue support of the effort, which began in FY 2006, to analyze structural variation in the human genome and to determine the contribution of structural variants to human diseases. The Institute also will continue to fund meritorious investigator-initiated applications submitted in response to announcements that encourage new technologies and new approaches to the analysis of genetic variation, and the role that genetic variation plays in the determination of human disease, disease susceptibility, and environmental sensitivities.
FY 2009 Level: $15.000 million
FY 2010 Level: $7.500 million
Change - $7.500 million
The 1000 Genomes Project is an ambitious effort to sequence the genomes of at least a thousand people from around the world to create the most detailed and medically useful picture ever of human genetic variation. The new genomes will provide a view of biomedically relevant DNA variations at a resolution unmatched by current resources. Researchers will use the catalog developed by the project for many future studies of people with particular diseases. Data from the 1000 Genomes Project will be swiftly available to the scientific community through freely accessible public databases, while simultaneously ensuring participant privacy and protection.
The 1000 Genomes Project builds on the human haplotype map developed by the International HapMap Project, but will provide a much more comprehensive view. It aims to find almost all the variants in the genome, including those that contribute to disease risk. The 1000 Genomes Project will map not only the single-letter differences in people's DNA, called single nucleotide polymorphisms (SNPs), but also will produce a high-resolution map of larger differences in genome structure called structural variants, which are rearrangements, insertions, deletions, or duplications of DNA segments. The importance of these variants has become increasingly clear with surveys completed in the past 18 months that show these differences in genome structure may play a role in susceptibility to such conditions as mental retardation and autism.
The project includes large-scale implementation of several new sequencing platforms. Using standard DNA sequencing technologies, the effort would likely cost more than $500 million. However, the cost is expected to be far lower — $30 million to $50 million — due to the project's pioneering use of new sequencing technologies.
Computational Genomics
As the speed of genotyping and DNA sequencing increase (accompanied by a continued decrease in their costs), the rate of data production will increase even more rapidly. The NHGRI supports a number of efforts in computational genomics research to continue development of database technology and computational methods critical to genome-wide studies.
The NHGRI will continue its support for genomic databases, an essential resource utilized worldwide to accelerate biomedical research. Ongoing and planned program announcements encourage development of new technologies and new approaches to the emerging issue of how to make the enormous amount of data generated by large-scale, genomic studies available to the broad research community and how to analyze such large datasets.
Budget Policy: The FY 2010 budget estimate for Computational Genomics programs is $47.014 million, a change of $628 thousand or 1.4 percent over the FY 2009 Estimate. Activity in Computational Genomics will remain essentially constant, maintaining the proportion of the NHGRI extramural budget devoted to this area. In FY 2010, the NHGRI will continue its support for the essential biomedical research resource represented by genomic databases. The Institute also will continue to fund meritorious investigator-initiated applications submitted in response to announcements that encourage new technologies and new approaches to the rapidly emerging issue of public access to large genomic datasets.
Technology Development
The mission of the NHGRI's technology development programs is to make DNA sequencing and other genomic analyses faster and more cost effective for use in both medical research and health care. The cost of DNA sequencing has fallen dramatically, more than 50-fold, over the past decade and continues to fall. The ability to sequence an individual genome inexpensively would not only further biomedical research, but would enable health care professionals to tailor diagnosis, treatment, and prevention strategies to each person's own genetic profile.
The NHGRI-supported grants were instrumental in the development of three new sequencing instruments now on the market and one more nearing market introduction. In addition, three firms that have pioneered development of new sequencing technologies have recently joined the international effort to build the most detailed map to date of human genetic variation as a tool for medical research, the 1000 Genomes Project (see above).
Grants supporting the creation of novel tools and technologies show promise to reduce further the cost of sequencing a human-sized genome, from the cost of ~$10 million within the past two years to $100,000 within the next two years. Another set of more than two dozen grants fund investigators who are developing breakthrough technologies that should make it possible to sequence a human genome for $1,000 within several years. In FY 2008, the NHGRI funded eight investigator teams to develop revolutionary technologies that would make it possible to sequence a genome for $1,000 or less, as well as three investigators developing nearer-term technologies to sequence a genome for $100,000.
Budget Policy: The FY 2010 budget estimate for technology development is $48.485 million, an increase of $951 thousand or 2.0 percent over the FY 2009 Estimate. The NHGRI will continue in FY 2010 its ground-breaking efforts to reduce the cost of DNA sequencing to the point at which the technology will be a widely disseminated research tool and a tool for individual healthcare. The Institute also will continue to fund meritorious investigator-initiated applications submitted in response to announcements that encourage the development of new technologies for biomedical and translational research.
FY 2009 Level: $22.436 million
FY 2010 Level: $24.212 million
Change +1.776 million
DNA sequencing costs have fallen dramatically over the past decade, fueled in large part by tools, technologies, and process improvements developed as part of the successful effort to sequence the human genome. Subsequently, the NHGRI launched programs to accelerate the development of sequencing technologies. Significant progress has been made towards the goal of producing high quality whole genome sequence for $100,000. The NHGRI's ultimate vision is to cut the cost of whole-genome sequencing of an individual's genome to $1,000 or less, which will enable sequencing as part of routine medical care. The imperative to collect large numbers of additional genome sequences is strong, but the cost has been much too high, motivating this technology-development thrust. The NHGRI is committed to medical sequencing for transitional "bench to bedside" applications – to establish links between specific changes in gene sequence and the many diseases that affect humans. The ability to sequence an individual genome inexpensively would not only further biomedical research, but also enable health care professionals to tailor diagnosis, treatment, and prevention strategies to each person's own genetic profile.
NHGRI-supported grants were instrumental in the development of three of the four new sequencing systems that recently reached the market. In FY 2008 alone, the NHGRI awarded more than $20 million in grants to develop innovative sequencing technologies inexpensive and efficient enough to sequence a person's DNA as a routine part of biomedical research and health care. Substantial technical advances stimulated by the first five years of investment may enable commercialization of yet another generation of sequencing systems in less than five years, to achieve the $1000 genome goal.
Other Basic Genomics
Multi-investigator, interdisciplinary research teams are crucial to develop novel and innovative genomic research projects and to foster the wider application of comprehensive, high-throughput genomics methods to the study of human biology and disease, using and expanding the data sets and technologies developed by the Human Genome Project.
Started in FY 2001, the NHGRI's Centers of Excellence in Genomic Science (CEGS) program supports the formation of such teams and also provides focal points for providing education and training about genomic research opportunities to members of under-represented population groups. In FY 2007, the NHGRI announced grants to establish a new CEGS focused on viral infections and renew support of a CEGS studying vertebrate diversity.
Budget Policy: The FY 2010 budget estimate for other basic genomics programs is $54.225 million, an increase of $91 thousand or 0.2 percent over the FY 2009 Estimate. In FY 2010, the NHGRI will continue to support the CEGS program in its efforts to stimulate highly innovative research approaches that will substantially advance genomic approaches to the study of a biological problem, and to foster the wider application of comprehensive, high-throughput genomics methods to the study of human biology and disease. The Institute will also continue to fund meritorious investigator-initiated applications that will increase the ability of genomics to have a major impact on the progress of biomedical and translational research.
Translational Genomics
The NHGRI is strongly committed to translating the information gleaned from studies of genomic function and variation into clinical applications. Diseases arise from a complex interplay between genes and environment; therefore, DNA variations, epigenetic factors, and external factors acting "on" the genome must all be considered in diagnosing and treating patients. Understanding this interplay will truly revolutionize our approach to health and health care, allowing not only much more accurate prediction of disease, but, ultimately, individual-based disease prevention.
In FY 2007, the initial projects of the Genes, Environment and Health Initiative (GEI), a collaboration with the National Institute for Environmental Health Sciences (NIEHS), were funded to identify and understand the interactions of environmental exposures with specific genetic variation. These included eight genome-wide association studies (focusing on addiction, birth weight, coronary heart disease, dental caries, lung cancer, oral clefts, prematurity and type 2 diabetes), two genotyping centers, a coordinating center, and more than 30 environmental technology projects. Another "bench to bedside" translational research project is an innovative study, in collaboration with the National Heart, Lung and Blood Institute (NHLBI), to evaluate the use of genetic variants to personalize the dosing of a commonly-used and potentially risky medication, Coumadin.
In FY 2008, the NHGRI announced grants expected to total about $31.000 million over the next four years for research aimed at gaining a better understanding of how specific genetic variants act to influence the risk of diabetes, heart disease, cancer and other common diseases. Scientists have already discovered more than 25 genetic variants in 18 genes connected to cholesterol and lipid levels.
Budget Policy:The FY 2010 budget estimate for translational genomics is $25.516 million, an increase of $909 thousand or 3.7 percent over the FY 2009 Estimate. The NHGRI is continuing to expand activity in the area of translational genomics, as the application of advances in genomics to problems of human health has a very high programmatic priority.
Ethical, Legal, and Social Implications
As the use of genetics and genomics in translational and clinical studies increases, the importance of addressing the ethical, legal, and social implications (ELSI) of the results of genetic and genomic research continues to grow as well. The NHGRI addresses such issues through its ELSI Research Program and through public consultation and community engagement that identifies and responds to culturally specific concerns and gives participating communities input into research, importantly including the informed consent and sample collection processes.
In FY 2004, the NHGRI launched an initiative to address the challenges of ELSI research related to the use of genetics and genomics in translational and clinical studies, the Centers of Excellence in ELSI Research (CEERs) program. The CEERs are charged with: 1) fostering the multi-disciplinary approaches necessary to make advances in understanding the issues that will be raised by progress in genomic science, 2) conducting ELSI research to inform the development of research, health, and public policies and practices and, 3) training the next generation of ELSI researchers. In FY 2008, the NHGRI established two new centers focused on the ELSI issues surrounding large-scale genomics research and emerging genetic technologies.
Budget Policy: The FY 2010 budget estimate for the ELSI program is $19.146 million, an increase of $777 thousand or 4.2 percent over the FY 2009 Estimate. The ELSI budget is legislatively mandated at 5.0 percent of the total NHGRI extramural budget. In FY 2010, the NHGRI will continue to support the ELSI research program in its efforts to anticipate and address the social, legal, and ethical issues that will arise from new information about the human genome and the genetic contribution to human disease, and new approaches to applying that information to the improvement of human health.
INTRAMURAL RESEARCH
NHGRI intramural researchers continue to focus on the genetic components of both rare and common disorders. As an example, a team of researchers led by NHGRI investigators recently completed the most comprehensive look to date at genetic risk factors for type 2 diabetes, identifying at least four new genetic variants associated with increased risk of diabetes and confirmed existence of another six. These findings boosted to at least ten the number of genetic variants confidently associated with increased susceptibility to type 2 diabetes — a disease that affects more than 200 million people worldwide. These investigators are now using contemporary genomics technologies, including powerful new methods for sequencing DNA, to identify the specific variants causing the increased risk for diabetes; this should yield new insights into the disease that may lead to new therapeutic avenues. Other research performed within the institute continues to have a profound impact on our understanding of more rare genetic disorders.
The NHGRI Division of Intramural Research plans to continue increasing its focus on translational research in FY 2010. The recently established Office of Translational Research, which is intended to encourage collaborations between basic scientists and clinical investigators, is already facilitating the translation of promising laboratory discoveries into clinical research studies. One notable advance in this area is the recent launching of a transdisciplinary program to characterize fully the complete set of microbes growing on human skin (the skin microbiome). This project is capitalizing on powerful new genomic technologies that allow detection of microbes that otherwise cannot be grown in the laboratory; these microbes are highly relevant to human health and to skin diseases.
Two clinical genomics initiatives launched in FY 2007 are now in full stride. The first, called ClinSeq, is a pilot study aimed at developing the technologic and procedural infrastructure to facilitate large-scale medical sequencing in a clinical research setting. The second, called the Multiplex Initiative, is a research study intended to provide and evaluate patients' reactions to genetic susceptibility testing for several common health conditions, such as cardiovascular disease and osteoporosis. These initiatives are providing a foundation for studies in genetic-based personalized medicine, an area that is becoming increasingly important in light of the proliferation of direct-to-consumer genetic testing services.
In FY 2008, the NIH launched the Undiagnosed Diseases Program (UDP) — a clinical research program that aims to provide answers to patients with mysterious conditions that have long eluded diagnosis. Representing a partnership between the NIH Office of Rare Diseases Research and the NHGRI, but involving dozens of health professionals at the NIH Clinical Center, the initiative focuses on the most puzzling medical cases referred to the NIH by physicians across the nation. Nearly 1,000 inquires have already been evaluated for potential participation in the program. More than 25 patients were enrolled in the program in FY 2008.
In FY 2008, the NHGRI Intramural Program recruited a world-class African-American researcher who studies the genetics and genomics of health disparities. He is the founding Director of the Center for Research on Genomics and Global Health, a new trans-NIH research entity that opened in the third quarter of FY 2008.
Budget Policy: The FY 2010 budget estimate for Intramural Research is $104.148 million, an increase of $1.539 million or 1.5 percent over the FY 2009 Estimate. This increase involves four areas.
- The recruitment of new Tenure-Track Investigators that will arrive at the end of FY 2009 following rigorous searches and recruitments.
- The addition of personnel to strengthen our translational and clinical research programs. This will include the recruitment of additional physician-scientists and other health professionals with expertise in clinical research, including some who will join the Office of Translational Research. It also includes continued growth of our flagship intramural clinical genomics projects, ClinSeq and Multiplex. Also, significant personnel growth will occur to support the recently launched Undiagnosed Diseases Program.
- Continued acquisition and implementation of new technologies for performing large-scale DNA sequencing. Genomics is currently seeing major growth in terms of new methods for obtaining very large amounts of DNA sequence data at lower and lower costs. The NHGRI Intramural Program will continue to implement these powerful 'next-generation' DNA sequencing technologies in FY 2010, along with the accompanying bioinformatics and computational infrastructure that they require. These new technologies are increasingly being applied to clinical research projects.
- Continued growth of our chemical genomics program, with its world-class expertise and experience in high-throughput screening of chemicals for biological function. These efforts include expansion into the study of inhibitory RNA molecules, and will increasingly involve both basic and clinical research applications.
RESEARCH MANAGEMENT AND SUPPORT
The NHGRI's Office of the Director, part of the RMS program, oversees the operation of the institute and includes a number of component parts. Major ongoing initiatives for which the Office of the Director provides key leadership and financial support include National DNA Day, the U.S. Surgeon General's Family History Initiative, and the development of genetics education resources for health professionals. DNA Day is an annual opportunity to educate students about genetics and genomics and to use this cutting edge field to spark their interest in science. The NHGRI staff collaborates with researchers, advocacy organizations (both professional and lay), and educators from formal and informal education institutions to reach students across the country. Outreach is achieved through classroom visits, professional development opportunities for educators, and a live web-based chatroom where students can post questions to the NHGRI researchers and staff that are answered in real-time. The U.S. Surgeon General's Family History Initiative is a coordinated multi-agency effort to encourage all American families to learn more about their family health history and to employ it in preventive health care. To make gathering this information easier, an improved version of the online web based tool, "My Family Health Portrait," as well as paper versions of the tool have been made available for download. To expand the initiative's reach and public benefit, the NHGRI continues to collaborate across federal agencies to enhance the family history tool's capabilities and to engage in demonstration projects to develop evidence regarding the tool's utility. Projects to promote the public's awareness and participation in the initiative will also be pursued. Finally, the NHGRI is taking a leadership role in facilitating the development, pilot testing, and dissemination of interdisciplinary web-based genetics educational resources for health professional groups, as well as community-based and public health organizations.
Budget Policy: The FY 2010 budget estimate for research management and support is $22.524 million, an increase of $387 thousand or 1.7 percent over the FY 2009 Estimate. In FY 2010, the NHGRI plans to continue to develop ongoing initiatives for which the Office of the Director provides leadership and financial support. Such programs within the Office of Policy, Communication, and Education include National DNA Day, the U.S. Surgeon General's Family History Initiative, and the development of genetics education resources for health professionals. In addition, the NHGRI is enhancing the Risk Management Program, which includes business process reengineering, setting up new procedures and tools to ensure our continued prudent use of RMS funds. RMS funds will be used to continue funding the activities mentioned above to support the infrastructure that allows the NHGRI to pursue and achieve its mission.
NIH COMMON FUND ROADMAP INITIATIVES
The NHGRI is the lead Institute for the Connectivity Map supported through the NIH Common Fund. In addition, the NHGRI is a co-lead for the Molecular Libraries initiatives, including:
- NIH Chemical Genomics Center
- Cheminformatics Computing Centers (virtual synthesis, virtual screening, other applications, and R&D on new tools), and
- Robotics/Instrumentation Technology Development, also supported through the NIH Common Fund
Finally, the NHGRI also co-leads the new Human Microbiome Project, also supported through the NIH Common Fund.
Budget Authority by Object
|
Salaries and Expenses
|
Authorizing Legislation
| ||||||||||||||||||||||||||||||||
Appropriations History
|
1 Reflects enacted supplementals, rescissions, and reappropriations.
2 Excludes funds for HIV,/AIDS research activities consolidated in the NIH Office of AIDS Research.
Detail of Full-Time Equivalent Employment (FTEs)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Detail of Positions
|
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research.
New Positions Requested
| |||||||||||||||||||||||||||||||||||
Last Reviewed: May 19, 2012
