How tumors differ from each other - either in different organs or within the same organ - can yield useful clues about prognosis and treatment. Ultimately, that knowledge may lead to precision medicine, where a doctor is not just treating a tumor, but tailoring treatment to the patient's specific tumor. A massive new analysis of tumors, published online April 17 in Epigenetics & Chromatin, is leading medicine closer to these goals.
Like all roads leading to Rome, there are many paths for a cell to become cancerous. One of these roads involves how the cell reads the epigenetic code, the "other genetic code" that sits atop a person's DNA. In every cell, DNA is tagged with chemical groups - like flags on the DNA - that make up the epigenetic code and act as guideposts. These flags help the cell's machinery know when to activate or inactivate a gene. In healthy cells, these flags are correctly placed and genes are expressed in the right proportions. However, in many tumor cells the flags are incorrectly applied.
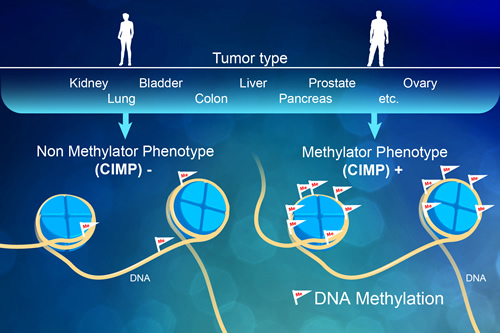
One form of incorrect DNA flagging occurs when extreme amounts of otherwise healthy methyl chemical groups, which act like on/off switches for a gene, are incorrectly added to many places on the cell's DNA. So, instead of a cell chugging along with its natural, biological jobs, these newly added methyl groups cause the cell to act erratically, leading to the cell expressing genes at the wrong levels, or not at all. This generally disrupts the normal flow of information in the cell. Importantly, these flags can turn off key defensive genes that help protect the cell from becoming cancerous and disrupt other processes. When this occurs, it's called "CpG Island Methylation Phenotype," or CIMP for short.
"When the chemical flags are placed on the gene inappropriately, the cell can lose its natural defenses, which can then lead to diseases like cancer," said Laura Elnitski, Ph.D., a senior investigator in the Translational and Functional Genomics Branch at the National Human Genome Research Institute (NHGRI). "In a way, it's like losing control of a city - stoplights go haywire, garbage collection ceases and speed limits are ignored among other issues."
Researchers have found that tumors with CIMP are linked to different outcomes for different tumor types. For example, in breast cancers, CIMP is associated with a lower risk of malignant tumors and a higher chance of survival. But the same isn't true for prostate cancer, where it can indicate more aggressive cancers. Thus, the patient's status can help doctors gain a more detailed picture of the specific tumor and disease progression.
To better group tumors, doctors need a way to analyze the tumors for CIMP to sort out patients who are "positive" versus "negative." A team headed by Drs. Elnitski and Francisco Sánchez-Vega, a former post-doctoral research fellow in Dr. Elnitski's lab, undertook a comprehensive analysis of CIMP in over 5,000 tumor samples from 15 different cancer types, as well as almost two dozen cancer cell lines. They found that CIMP could be found in subsets of almost all types of cancer and represented an extreme difference from normal samples.
From these thousands of tumors, over 8,000 DNA flags could be used to define CIMP in the tumors. From this point, they winnowed down that pool of 8,000 flags to a small, robust set of only 89 markers that can accurately determine a patient's status in at least 12 tumor types. These findings should help further classify tumors, giving patients and practitioners alike valuable information. It may also enable diagnostics and treatments to move one step closer to precision medicine.
"In recent years, CIMP has been studied in tumors in a diverse set of organs, so we now know a lot of tumors have this. It's what we call a "pan-cancer" phenomenon, meaning it's something we see across many types of cancer," said Dr. Elnitski. "With this set of markers, we hope to understand what causes CIMP, to find out what's happening in a tumor at the molecular level and, more importantly, to help clinicians guide the care for their patients so they receive not only better care, but the right care."
To get to the promise of precision medicine - with its targeted therapies and treatments - the science of medicine must be able to classify illnesses into ever-smaller subgroups. So, a cancer diagnosis wouldn't tell a patient simply the type of cancer and the location, but also the molecular and epigenetic signatures of the patient's specific cancer. With this information, both prognosis and treatment will become more precise.
In the long term, this CIMP research may help other scientists uncover the genetic mechanisms behind this particular cancer-associated event. Eventually, those mechanisms may be exploited as targets for other therapies. In the short-term, this research provides a solid foundation to continue to study CIMP, as well as a proposed set of markers that could be used to create a clinical test to identify tumors with CIMP. If and when a test comes to fruition, doctors would have a better clinical picture of these cancers. This will enable them, hopefully, to tell worried patients more about their cancer, providing answers to questions that might help patients feel a bit more in control.
Read the Study
Francisco Sánchez-Vega, Valer Gotea, Gennady Margolin and Laura Elnitski. Pan-cancer stratification of solid human epithelial tumors and cancer cell lines reveals commonalities and tissue-specific features of the CpG island methylator phenotype. Epigenetics & Chromatin, 8:14. 2015. [Full Text]