The Direct-to-Consumer Genetic Testing (DTC-GT) Project Group of the Inter-Society Coordinating Committee for Practitioner Education in Genomics has created a DTC-GT Frequently Asked Questions (FAQ) resource designed for the general clinician who may see patients requesting guidance on DTC-GT. This FAQ is intended to help healthcare professionals understand the diverse landscape of DTC-GT, the benefits and limitations of these tests and how results of DTC-GT may affect their patients’ health, wellness and medical decision making.
Explore This Page
- Direct-to-Consumer Genetic Testing for Healthcare Professionals
- Possible DTC-GT Results
- DTC-GT and Adopted Adults
- DTC-GT and Pharmacogenomic Information
- DTC-GT Raw Data, Third-Party Interpretation Services and Data Privacy
- DTC-GT and Insurance Coverage
- Costs of Genetic Testing
- DTC-GT Information for Patients
- Genetic Counselors and Other Genetics Professionals
- Videos from Healthcare Professionals' Genomics Education Week
- Bibliography
- Authors and Reviewers
Direct-to-Consumer Genetic Testing for Healthcare Professionals
What is direct-to-consumer genetic testing?
Direct-to-consumer genetic tests (DTC-GT) are genetic tests sold directly to consumers to provide information about their genetic information (generally ancestry, some health traits and health risks) from a saliva sample. Ordering and return of results for DTC-GT typically does not involve health care professional engagement or the use of health insurance to cover the cost of testing. MedlinePlus Genetics gives an overview of DTC-GT for patients.1
Presentation by the ISCC-PEG DTC-GT Project Group
What are other types of genetic testing?2,3
Three main categories of genetic testing are currently available: Clinic-based, direct-to-consumer, and provider-mediated genetic testing (see table 1). Clinic-based genetic testing is ordered, interpreted and disclosed by a physician or other healthcare professional through a traditional healthcare professional-patient relationship. Standard-of-care approaches to clinic-based genetic testing involve a pre-test consultation to identify and document potential genetic risk factors, select the most appropriate test, and discuss the benefits and limitations of testing to facilitate informed consent from the patient. During a post-test visit, test results are disclosed and interpreted.
In recent years, multiple types of genetic testing products marketed directly to consumers have emerged. Compared to direct-to-consumer genetic tests (DTC-GT), provider-mediated genetic testing (PM-GT) engages a healthcare professional in a non-traditional role as part of the testing process. The professional’s involvement may be limited to placing the test order or approving the order for genetic testing with minimal interaction or discussion of the test with the consumer. This professional may be employed by the company that conducts the genetic test and may not know the consumer, or the healthcare professional may already care for the consumer.2
| Description | Who orders the test? | How is consent obtained before the test? | Who discloses the results? | |
|---|---|---|---|---|
| Direct-to- consumer genetic testing (DTC-GT) | Healthcare professional not involved | Consumer | Direct to consumer via written information | Report issued directly to consumer |
| Provider-mediated genetic testing (PM-GT)3 | Consumer-initiated with healthcare professional involvement | Consumer or healthcare professional | Direct to consumer with/without healthcare professional | Report issued directly to consumer with optional access to healthcare professional |
| Clinic-based genetic testing | Facilitated by healthcare professional | Healthcare professional | By healthcare professional | Facilitated by healthcare professional |
Table 1: Types of genetic testing
What is the history of DTC-GT?4–6
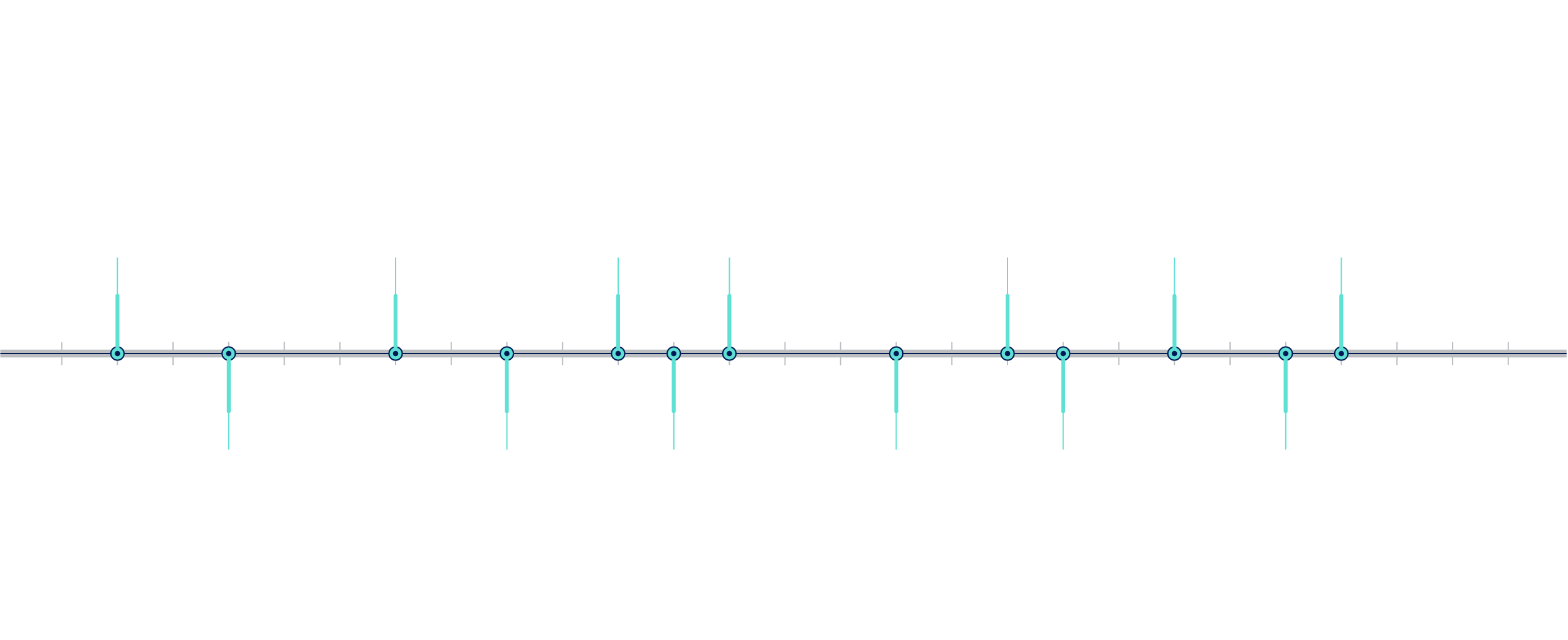
Figure 1: Timeline of direct-to-consumer genetic testing (DTC-GT). FDA = Food and Drug Administration; GAO = US Government Accountability Office.
Milestones in the Timeline of DTC-GT:
- 1996: A UK genetic testing company launched mail-order genetic testing. In the United States, genetic testing companies marketed BRCA gene testing directly to consumers via newspapers.
- 1998: A clinical genetic testing company launched a consumer advertising campaign on television, on radio and in magazines.
- 2001: A nutrigenetics company in the United Kingdom marketed genetic tests to consumers directly via their website.
- 2003: The Human Genome Project was completed.
- 2005: The first DTC companies were founded.
- 2006: The US Government Accountability Office (GAO) investigated DTC-GT companies.
- 2010: GAO released a report indicating that these companies made medically unproven disease predictions.
- 2007: DTC-GT was offered at $1,000.
- 2010: The Food and Drug Administration (FDA) initiated regulatory proceedings against DTC-GT companies.
- 2012: DTC-GT prices dropped to $99.
- 2013: The FDA sent cease and desist letters to DTC-GT companies. In response, some DTC-GT companies elected to end their testing services. Others modified their products as instructed by the FDA, then worked closely with the FDA to ensure that existing and newly developed testing products met their standards.
- 2015: The FDA approved DTC-GT carrier screens.
- 2017: The FDA approved DTC-GT for certain health-related conditions.
- 2018: more than 26 million consumers had contributed their DNA to commercial databases.
- 2020: the FDA granted a DTC-GT company clearance for a pharmacogenetics report for interpretive drug information for two medications — clopidogrel and citalopram — removing the need for confirmatory testing.
What are the benefits of DTC-GT from a healthcare standpoint?
While DTC-GT does not test for most genetic traits or diseases, DTC-GT can provide information about potential risk for some genetic conditions or for being a carrier for certain genetic diseases. Knowing this information can remove some of the fear and stigma that may be associated with genetic testing and with discussions around family health history.
Some DTC-GT options can give consumers a better sense of personal health agency, allowing for a more proactive approach to their health. After people share their DTC-GT results with their healthcare professionals, they may be motivated to consider the professionals’ screening and intervention recommendations.
DTC-GT often provides results for specific variants more quickly and can be less expensive than some clinical genetic tests ordered through a healthcare professional. This is especially true in cases where people do not have adequate health insurance or established relationships with healthcare professionals, and are concerned about waiting times. Testing is non-invasive and does not require a blood draw.
Consumers are not required to share their results from DTC-GT with their insurance professionals, which offers an additional level of privacy. In some cases, a consumer’s desire to be a part of research is a motivator to have DTC-GT because they can opt into research studies being performed by the DTC-GT company.
What are the limitations of DTC-GT?
One of the major limitations of DTC-GT is that it often does not test for all pathogenic or disease-causing variants for a particular condition, so the results cannot be used to rule out a condition. In addition, some consumers may be interested in a particular condition that DTC-GT does not cover. Even if DTC-GT shows a potentially actionable result (e.g., a test detects a BRCA1 pathogenic variant), these results need to be confirmed with a clinical genetic test; therefore, DTC-GT may add an unnecessary step to the process, as well as additional time and cost.
Some DTC-GT companies may test for conditions or traits that do not have confirmed clinical utility or have no confirmed gene to phenotype correlation (e.g., genetic testing to see which vitamins a person should take).
Another limitation is that DTC-GT is often completed without traditional genetic counseling, risk assessment or informed consent to confirm that the consumer fully understands the implications of the possible results. DTC-GT may give patients data that are overwhelming, non-actionable or distressing without the support of a qualified healthcare professional.
For ancestry results, DTC-GT may reveal a genetic relationship to biological relatives that is unexpected and possibly distressing. These tests may uncover ancestral origins that may be previously unknown. In addition, the databases of DTC-GT companies do not cover all ethnic populations equally; therefore ancestry results for underrepresented minority populations may be inaccurate.
More recently, some DTC-GT companies have been in the media for allowing law enforcement to access their databases to solve crimes.7 As a result, consumers may have privacy and safety concerns. Additionally, depending on the DTC-GT company, an individual’s DNA may be used for research or commercial purposes, and consent for each use may not have been expressly given or understood.
Do the methodologies for DTC-GT and clinic-based testing differ?
DTC-GT for health conditions and carrier status is often performed by single nucleotide polymorphism (SNP) arrays (See How does DTC-GT use SNP arrays?) A few types of DTC-GT also use next-generation sequencing, but these are for more specialized tests. In contrast, DTC-GT for ancestry uses SNP arrays, short tandem repeats (STR) testing or region sequencing. Many of the DTC-GT used today in 2021 only look at a small slice of a person’s genomic information to make estimates about certain health traits, risks or ancestry.
Clinic-based genetic testing typically involves more thorough genetic testing methods including SNP arrays, next-generation sequencing, copy number analysis or methylation analysis. The methodology used depends on the genetic condition. Results are confirmed and interpreted by the clinical lab within the context of published medical guidelines and by the healthcare professionals with consideration of the patient’s personal and family health history.
Even when DTC-GT uses more comprehensive methodologies, the test reports only include a predetermined set of variants.
Clinical genetic testing labs must have established quality standards, such as Clinical Laboratory Improvement Amendments (CLIA) certification and College of American Pathologists (CAP) accreditation. Although a few DTC-GT companies do have these credentials, they are not required to have them. Some DTC-GT labs may have FDA approval, but the FDA states, “Results obtained from the tests should not be used for diagnosis or to inform treatment decisions. Users should consult a healthcare professional with questions or concerns about results.”8
DTC-GT is often performed by SNP array. What kind of results do SNP arrays in DTC-GT provide?
The number and location of tested SNPs varies among DTC-GT companies. Some of the SNPs on the array have clinical relevance and may be used to establish carrier status for genetic conditions. Other SNPs may suggest genetic conditions or health risks in the tested individual or may be relevant for medication selection and dosing.
Certain SNPs on the array are used to determine a person’s ancestry. Differences in SNP frequencies between groups with different ancestry can help track the human family tree. Computer algorithms analyze a consumer’s SNP results and determine the most likely ancestral origin for each section of the DNA. With 100,000 to 1 million markers, these estimates are usually accurate, but they rely heavily on pre-existing data. Minority populations may be mis-identified by DTC-GT if DNA sequences from their group are not available for comparison.
Possible DTC-GT results
Results from DTC-GT may come back as a “genetic variant detected.” A genetic variant is an alteration in the sequence compared to the reference genome (a database representative of the “typical” human genome). When a variant is found, it may or may not be clinically actionable because the DTC-GT standards and guidelines for the interpretation of the variant are different from those of clinical genetic testing.11 Some DTC-GT results can have implications for a patient’s and their family members’ health and wellness.
Patients are encouraged to consider genetic counseling or other genetics professionals if DTC-GT results show they are a carrier for an inherited genetic condition, have a high-risk or actionable pathogenic variant or want to know about a health trait. During this consultation, clinical genetic testing may be offered to validate a DTC-GT result or to explore other personal and family health history.
If patients are pregnant or considering future pregnancy, they may consider seeing a prenatal genetic counselor or other genetics professional to discuss the results of their DTC-GT, consider clinical genetic testing to confirm results and conduct a thorough review of family health history and assessment of potential risk to a pregnancy.
What is DTC-GT carrier testing?
Carrier testing is used to identify individuals who carry one copy of a pathogenic variant that, when present in two copies, causes a genetic disorder. Carriers often do not show symptoms of a condition but can pass their copy of a pathogenic variant to their children who could be affected by a genetic condition, such as cystic fibrosis.
What to do if a patient has a positive DTC-GT carrier result?
- If a patient has a positive carrier test result, consider referring them to a genetic counselor or other genetics professional.
- A genetics professional will discuss clinical genetic testing to confirm variants and address potential risks to biological family members, if any.
- The carrier patient and close biological family members should be offered education about their carrier status and inheritance of the variant.
- Preconception or prenatal testing can be considered if the patient is pregnant or considering future pregnancy.
What types of disease risk and health results does DTC-GT provide?
Some results from DTC-GT estimate the risk that the consumer will develop several common diseases by testing for low-risk genes (e.g., variants in HLA-DQA1 and HLA-DQB1 genes for celiac disease, GBA gene for Parkinson disease and APO gene for Alzheimer disease). DTC-GT can detect pathogenic variants in moderate-risk genes like HFE, which is associated with hemochromatosis. Some DTC-GT tests can also detect pathogenic variants in high-risk genes, like BRCA1 and BRCA2, that more definitively predict an increased risk of breast and ovarian cancer.
When DTC-GT testing does not detect high-risk or actionable variants, patients may have a false sense of security and believe they are not at risk. However, they may still have a genetic variant that DTC-GT did not test for or report. Healthcare professionals could consider a discussion about how DTC-GT testing does not look for all pathogenic variants or genetic rearrangements related to risk for a disease. If there is a personal or family history that indicates a genetic syndrome, healthcare professionals could consider referring the patient to a genetic counselor or other genetics professional to discuss clinical genetic testing.
What actions can healthcare professionals take for varying levels of disease risk identified by DTC-GT?
High-risk and moderate-risk examples
A DTC-GT report shows a pathogenic variant in BRCA1 associated with hereditary breast and ovarian syndrome (HBOC), or a DTC-GT report shows two copies of disease alleles associated with hereditary hemochromatosis.
- If a patient is found to have a high-risk variant detected through DTC-GT, they should be referred to a genetic counselor or other genetics professional.
- In the meantime, clinical evaluation including a three-generation family history can be performed to confirm the diagnosis as appropriate in the tested individual
- A genetics professional will discuss clinical genetic testing to confirm variants and address potential risks to biological family members, if any.
- If variants are confirmed, the patient and close biological family members should be offered education, increased screening, treatments and interventions as appropriate.
A low-risk example
A DTC-GT report shows an increased risk for late onset Alzheimer disease, but the variant detected only contributes a portion of the total risk to the disease.
- Conditions such as Alzheimer disease are caused by multiple factors. These factors include variants in multiple genes, a patient’s lifestyle and their environment. Because of this, a single gene variant is not likely to be actionable and is not clinically useful at this time.
- If a patient is concerned about the variants detected through DTC-GT in conjunction with a family history of the condition, consider referring them to a genetic counselor or other genetics professional.
What are health-trait and lifestyle results from DTC-GT?
These results give information and recommendations about lifestyle factors — such as nutrition, fitness, weight loss, skincare and sleep — based on variations in DNA. However, due to the complexity of these factors, the role genetics plays in relation to these factors is difficult to ascertain and the usefulness of these recommendations is unclear in most cases. Trait and lifestyle recommendations as a result of DTC-GT can be difficult to interpret and are often indirectly linked to a trait.
Health-trait example:
A patient’s report shows that they cannot digest certain foods.
- Before patients alter their health, diet or fitness practices, healthcare professionals may want to investigate the patient’s clinical history to determine if the DTC-GT result is in alignment with the clinical findings.
- If there is alignment, consider referring the patient to the appropriate specialist for further investigation and management.
DTC-GT and Adopted Adults
What if my patient is adopted or has little family history?
Adoptees often do not have records or knowledge of genetic family health history due to either missing or untranslated records in intercountry adoption or lack of access to birth records in domestic adoption. Even if an adoptee has contact with a biological relative, their contact may be sporadic and the adoptee may not be able to piece together a complete family health history.
The use of DTC-GT is one of the few ways adoptees can open doors to their genetic health history. While not a traditional care model, DTC-GT allows adoptees a way to begin to understand their genetics and take action based on their results. These results are often the only glimpse that adoptees have of their biological makeup and can be meaningful to them even if their results are inconclusive.
How do I handle DTC-GT results for an adoptee?
If an adoptee is found to have a positive carrier test result, disease risks or other health result, consider referring them to a genetic counselor or a genetics professional.
A genetics professional will discuss clinical genetic testing to confirm if variants are pathogenic and address potential risks, if any. The adoptee may be offered education, increased screening, treatments and interventions as appropriate.
Results from DTC-GT may have an impact on adoptees and their biological relatives, if any are known. Healthcare professionals are encouraged to discuss how DTC-GT results may affect biological children or close relatives. A discussion on relatedness and inheritance may also be appropriate, as DTC-GT may reveal distant relatives.
Preconception or prenatal testing can be considered if the adoptee desires to be or is pregnant. They may want to consider seeing a prenatal genetic counselor or other genetics professional to discuss the results of their DTC-GT, consider clinical genetic testing to confirm results and review inheritance and risk to a pregnancy.
What if an adoptee is considering DTC-GT for health information?
If an adoptee does not meet criteria for clinical screening or medical interventions based on personal health history and is motivated to explore genetic testing for hereditary conditions, consider referring them to a genetic counselor or genetics professional to discuss clinical genetic testing and patient self-pay options.
In addition, consider discussing the limitations of DTC-GT for health information; if adoptees test negative for disease risks and health results via DTC-GT, they may have a false sense of security because they may carry a pathogenic variant not included in DTC-GT. If they test positive for a genetic variant related to disease, they should confirm these results with clinical genetic testing.
What if my patient was conceived using donor eggs or sperm and wants to use DTC-GT to obtain health information?
DTC-GT is not comprehensive and should not be used to obtain health information. DTC-GT results may also violate donor agreements for anonymity. Instead, consider referring the patient to a genetic counselor or other genetics professional to discuss carrier screening or genetic testing for hereditary conditions.
Many donor banks screen eggs and sperm for common hereditary conditions, but not all companies test for the same ones and the tests are not considered comprehensive or clinically validated. If a patient is interested in their past screening, consider recommending that they reach out to their donor egg bank or sperm bank to discuss what genetic screening or testing was performed.
DTC-GT and Pharmacogenomic Information
Can DTC-GT provide pharmacogenomic information?
DTC-GT may provide pharmacogenomic information. These tests may help identify individuals who are unlikely to respond to a particular medication, need dose adjustment, or are at an increased risk for adverse effects.12 In October 2018, the U.S. Food and Drug Administration (FDA) approved the first DTC-GT for pharmacogenomics. Consumers will likely have access to additional DTC-GT pharmacogenomic tests in the near future.
Can I use DTC-GT pharmacogenomic test results to select optimal medication therapy or adjust the doses of my patients’ medications?
In general, healthcare professionals should not use DTC pharmacogenomic test results to change a patient’s medication therapy. When the FDA approved the first DTC pharmacogenomic test, they did so with the caveat that these results should not be used to make medical decisions without confirmatory clinical testing. However, in 2020, the FDA granted a single DTC-GT company clearance for the CYP2C19 gene report to provide gene-based prescribing information for clopidogrel and citalopram, removing the need for confirmatory testing for this gene only.8 In the future, the FDA may grant similar clearance for other genes. In the meantime, if the healthcare professional would like to use other DTC pharmacogenomic test results to guide therapy, they should order confirmatory clinical pharmacogenomic testing by a clinical laboratory before making medication adjustments. Healthcare professionals should counsel their patients not to use DTC pharmacogenomic results to modify or discontinue their medication regimen on their own.
What are the limitations of DTC pharmacogenomic tests?
At the time of writing, FDA-approved DTC-GT pharmacogenomic tests are limited to eight genes and 33 total variants that may impact drug response. These results may inform the decision to pursue additional testing. However, it is important to remember that the majority of DTC pharmacogenomic tests are not approved to be used directly to change prescribing; confirmatory clinical testing is needed. In addition, DTC pharmacogenomic tests may include fewer pharmacogenomic variants than clinical tests for certain genes, which could lead to differences between DTC pharmacogenomic test results and confirmatory clinical testing results. For example, if a patient does not have one of the variants that were tested, their result will be returned as normal (e.g., *1/*1). The patient may actually have a normal genotype, but it also might mean that the patient has a variant that was not tested. For example, there are more than 100 known variants for the gene CYP2D6. One FDA-approved DTC pharmacogenomic test includes 17 of those variants. Therefore, the patient could harbor a variant not included on the test that affects their CYP2D6 enzyme activity and their response to a drug, but their DTC-GT results could come back as normal.
What genetic variants have the FDA currently approved for DTC pharmacogenomic tests?
A limited number of pharmacogenomic variants (33 in eight genes) have been approved by the FDA for DTC-GT. A list of these variants can be found on the FDA website.18 These genes encode for drug metabolizing enzymes and drug transporters that have clinical utility in drug selection and/or dosing decisions.
What resources are available to help interpret and apply clinical pharmacogenomic test results to patient care?
The FDA provides gene-based prescribing information in certain drug labels. A complete list of pharmacogenomic variants included in FDA-approved drug labels can be found on the FDA website.
In addition, evidence-based clinical practice guidelines are available through the Clinical Pharmacogenetics Implementation Consortium (CPIC). These guidelines provide specific recommendations based on pharmacogenomic test results. Pharmacogenomic test results should always be interpreted in the context of other clinical variables that may influence drug response. Note that CPIC guidelines do not tell healthcare professionals when a pharmacogenomic test should be ordered; instead, they explain what to do if the patient already has the test results.
The Pharmacogenomics Knowledgebase (PharmGKB) is a searchable database of curated pharmacogenomic information, including primary literature, drug labeling from around the world and clinical practice guidelines from CPIC and other consortia. Clinicians may search for drugs, genes or drug/gene combinations.
Healthcare professionals may consider referring the patient to a pharmacist or genetics professional for further guidance. Some pharmacists have specialized training in clinical pharmacogenomics.
DTC-GT Raw Data, Third-Party Interpretation Services and Data Privacy
What should I tell my patient about raw data from direct-to-consumer genetic testing?
Raw data refers to the data file consumers may receive from their DTC-GT company. The data file consists of uninterpreted genotype information.2 Many of the variants in a consumer’s raw data are used for research by the DTC-GT company and often do not have enough data to classify any variant as pathogenic. However, consumers are curious about their raw data and may upload their raw data files to third-party interpretation services to help interpret the data.19 Using raw data to obtain additional analysis through third-party interpretation services can yield false positive results; results should be clinically confirmed before making any healthcare decisions. MedlinePlus Genetics provides excellent information about DTC-GT raw data.20
Third-party interpretation services 21
- High risk for false positives: Some of the third-party interpretation services claim to give consumers more details about their disease risks and traits.22 However, many of these services have provided false positives and unexpected information, both of which can cause unnecessary distress to consumers.
- Privacy concerns and few regulations: One of the most significant concerns with third-party interpretation services is the lack of informed consent and inconsistent privacy measures.21,23 Law enforcement has been known to search third-party databases for distant relatives of suspected criminals in order to locate the suspect.7 Additionally, there is little regulation of these services.
- Raw-data results still need clinical confirmation: If patients bring their raw data report to a healthcare professional, consider referring them to a genetic counselor or other genetics professional to discuss their findings and consider clinical genetic testing to confirm a suspected pathogenic variant.
How do direct-to-consumer genetic testing companies protect customer privacy?
The ways in which DTC-GT companies protect consumers’ privacy vary.20 Consumers should be aware of how the companies protect their genetic information and personal identifiers, and they should check to see if their genetic information might be used for research or advertising. Privacy policies and statements are located on the testing companies’ websites, but consumers may require time and assistance to find and understand the policies. For more information on privacy concerns, visit MedlinePlus Genetics.20
DTC-GT and Insurance Coverage
Can the results of direct-to-consumer genetic testing affect my patient’s ability to get insurance?19
The Genetic Information Nondiscrimination Act (GINA) of 2008 prohibits discrimination in health coverage and employment based on genetic information.24,25 The genetic information protected by the law includes family health history, the results of genetic tests (including direct-to-consumer genetic tests), the use of genetic counseling and other genetic services and participation in genetic research. Along with nondiscrimination provisions of the Health Insurance Portability and Accountability Act (HIPAA), GINA generally prohibits health insurers or health plan administrators from requesting or requiring genetic information of an individual or the individual’s family members (up to fourth-degree relatives), and from using it for decisions regarding coverage, rates or preexisting conditions. GINA does not provide protections for life, disability, or long-term care insurance.
For more information about GINA, see:
Costs of Genetic Testing
How much does DTC-GT cost?
DTC-GT prices vary depending on how many variants are examined, the type of results offered and the structure of the test (e.g., ancestry only, health and ancestry, health only or analysis and consultation with a genetic counselor). The price can range from under $100 to thousands of dollars. Because DTC-GT results are typically not considered diagnostic, health insurance companies often do not cover the cost of the test.
How much does clinical genetic testing cost?
When needing to confirm a DTC-GT result with clinical genetic testing, the patient should find out whether their insurance provider covers clinical genetic testing. For example, a patient may not meet the national cancer guidelines that recommend who should be offered genetic testing based on personal and family history of cancer; as a result, their insurance provider may not cover the cost of clinical genetic testing. However, many large clinical genetic testing companies offer a self-pay option, less expensive rates and financial assistance programs.
If the patient meets genetic testing guidelines, their insurance may have a high deductible, which may make the cost of testing unaffordable. In this scenario, the self-pay option or financial assistance program from a genetic testing company may offer a lower cost for the patient. For more information on insurance and costs, please visit MedlinePlus Genetics.26
DTC-GT Information for Patients
Where can patients find resources about DTC-GT?
MedlinePlus Genetics at the National Library of Medicine has a website with information targeted specifically to patients.
Where can I find resources about the diseases and traits reported by DTC-GT?27
Patient Resources
Professional Resources
Genetic Counselors and Other Genetics Professionals
Where can I find a genetics professional to refer a patient for a genetics consultation?
The National Society of Genetic Counselors (NSGC) provides information on how to find a genetic counselor nationwide for consultation in person or by phone.
The American College of Medical Genetics and Genomics (ACMG) offers a search form by type of specialist patients may need.
The ACMG also has a Find a Genetics Clinic tool.
The Genetic and Rare Disease Information Center has a toll-free phone number to help patients find specialists: (888)205-2311.
Videos from Healthcare Professionals' Genomics Education Week
Direct-to-Consumer Genetic Testing: Update and Cases
ISCC-PEG Direct to Consumer Genetic Testing (DTC GT) Project Group
June 5, 2023
Bibliography
- What is direct-to-consumer genetic testing? MedlinePlus Genetics
- Ramos, E. & Weissman, S. M. The dawn of consumer-directed testing. American Journal of Medical Genetics Part C: Seminars in Medical Genetics 178, 89–97 (2018).
- Chokoshvili, D., Vears, D. F. & Borry, P. Growing complexity of (expanded) carrier screening: Direct-to-consumer, physician-mediated, and clinic-based offers. Best Practice & Research Clinical Obstetrics & Gynaecology 44, 57–67 (2017).
- Regalado, A. More than 26 million people have taken an at-home ancestry test. MIT Technology Review.
- Allyse, M. A., Robinson, D. H., Ferber, M. J. & Sharp, R. R. Direct-to-Consumer Testing 2.0: Emerging Models of Direct-to-Consumer Genetic Testing. Mayo Clinic Proceedings 93, 113–120 (2018).
- Hogarth, S. & Saukko, P. A market in the making: the past, present and future of direct-to-consumer genomics. New Genetics and Society 36, 197–208 (2017).
- Kaiser, J. We will find you: DNA search used to nab Golden State Killer can home in on about 60% of white Americans. Science (2018) doi:doi:10.1126/science.aav7021.
- Office of the Commissioner. FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. FDA (2017).
- Fan, H. & Chu, J.-Y. A brief review of short tandem repeat mutation. Genomics Proteomics Bioinformatics 5, 7–14 (2007).
- Mitochondrial DNA. MedlinePlus Genetics.
- Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17, 405–424 (2015).
- Ramsey, L. B. et al. Prescribing prevalence of medications with potential genotype-guided dosing in pediatric patients. JAMA Netw Open 3, (2020).
- Chanfreau-Coffinier, C. et al. Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration pharmacy users. JAMA Netw Open 2, (2019).
- Mostafa, S., Kirkpatrick, C. M. J., Byron, K. & Sheffield, L. An analysis of allele, genotype and phenotype frequencies, actionable pharmacogenomic (PGx) variants and phenoconversion in 5408 Australian patients genotyped for CYP2D6, CYP2C19, CYP2C9 and VKORC1 genes. J Neural Transm (Vienna) 126, 5–18 (2019).
- Van Driest, S. L. et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther 95, 423–431 (2014).
- Kalman, L. V. et al. Pharmacogenetic allele nomenclature: international workgroup recommendations for test result reporting. Clin Pharmacol Ther 99, 172–185 (2016).
- The Pharmacogenomics Knowledge Base. PharmGKB.
- Office of the Commissioner. FDA authorizes first direct-to-consumer test for detecting genetic variants that may be associated with medication metabolism. FDA (2018).
- Can the results of direct-to-consumer genetic testing affect my ability to get insurance? MedlinePlus Genetics .
- How do direct-to-consumer genetic testing companies protect their customers’ privacy? MedlinePlus Genetics.
- Moscarello, T., Murray, B., Reuter, C. M. & Demo, E. Direct-to-consumer raw genetic data and third-party interpretation services: more burden than bargain? Genetics in Medicine 21, 539–541 (2019).
- Badalato, L., Kalokairinou, L. & Borry, P. Third party interpretation of raw genetic data: an ethical exploration. Eur J Hum Genet 25, 1189–1194 (2017).
- Phillips, A. Giving away more than your genome sequence?: privacy in the direct-to-consumer genetic testing space. 36.
- Genetic Discrimination. Genome.gov.
- The Genetic Information Nondiscrimination Act of 2008 | U.S. Equal Employment Opportunity Commission.
- How much does direct-to-consumer genetic testing cost, and is it covered by health insurance? MedlinePlus Genetics.
- Where can I read more about the diseases and traits covered in my direct-to-consumer genetic testing report? MedlinePlus Genetics.
Authors
Houriya Ayoubieh, M.D., FACMG
Assistant Professor,
Texas Tech University Health Sciences Center, El Paso, TX
Kathleen Blazer, Ed.D., M.S., CGC
Director, Cancer Genomics Education Program, City of Hope
Duarte, CA
Dyanna Christopher, M.P.H.
American Society of Human Genetics / National Human Genome Research Institute
Genetics and Education Fellow (2018)
Kathryn Garber, Ph.D.
Associate Professor, Emory University School of Medicine
Atlanta, GA
Roseann Gammal, PharmD, BCPS
Associate Professor, Massachusetts College of Pharmacy and Health Sciences
Boston, MA
Linda Ho, MSPP
Presidential Management Fellow
National Center for Advancing Translational Sciences
Katherine Hyland, Ph.D.
Professor, University of California San Francisco School of Medicine
San Francisco, CA
Kimberly Jacoby Morris, Ph.D.,
National Human Genome Research Institute
National Institutes of Health
U.S. Department of Health and Human Services
Bethesda, MD
Heewon Lee, M.S., CGC
Park Nicollet Frauenshuh Cancer Center
St. Louis Park, MN
Rachel Mills, M.S., CGC
Assistant Professor, University of North Carolina - Greensboro
Master of Science in Genetic Counseling Program
Greensboro, NC
Sarah Robbins Poll, Ph.D.
American Society of Human Genetics / National Human Genome Research Institute
Genetics and Education Fellow (2019)
Tracey Weiler, Ph.D.
Associate Professor, Herbert Wertheim College of Medicine
Florida International University
Miami, FL
Mylynda Massart, MD., Ph.D.
Assistant Professor, University of Pittsburgh School of Medicine
Pittsburgh, PA
Reviewers
Christine M. Formea, Pharm.D., BCPS
Associate Professor, Intermountain Healthcare
Salt Lake City, UT
Matthew Taylor M.D., Ph.D.
Professor, Director Adult Medical Genetics Program
University of Colorado Anschutz Medical Campus
Aurora, CO
Donna Messersmith, Ph.D.
ISCC-PEG Co-Chair
National Human Genome Research Institute
National Institutes of Health
The Inter-Society Coordinating Committee for Practitioner Education in Genomics (ISCC-PEG) provides a venue for individuals to collaborate and develop educational resources. The opinions expressed in this resource do not reflect the view of all ISCC-PEG members, the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Last updated: June 14, 2023
