The Big Picture
-
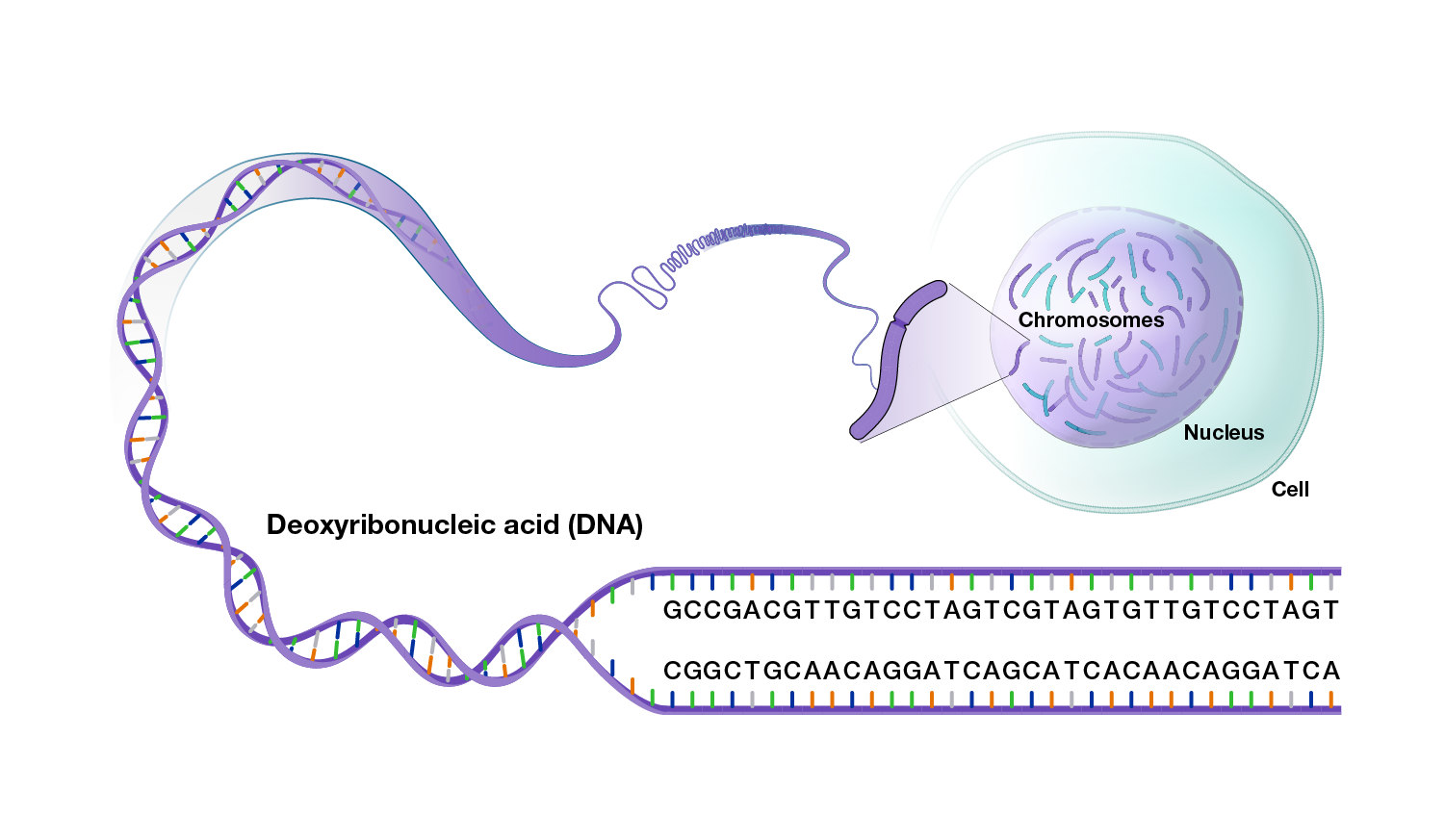
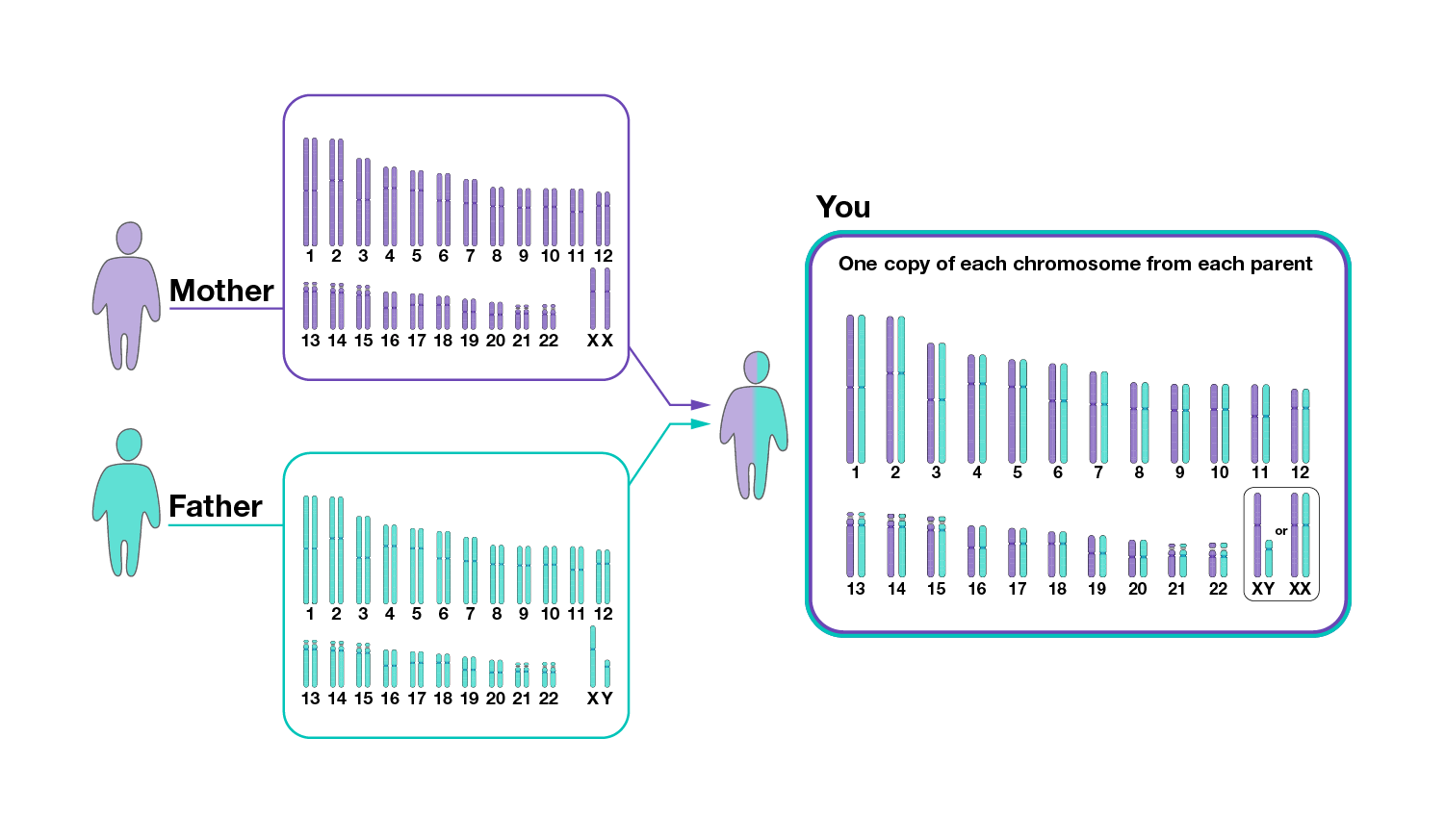
Genomic variation reflects the differences in a person’s DNA compared to other peoples’ DNA.
-
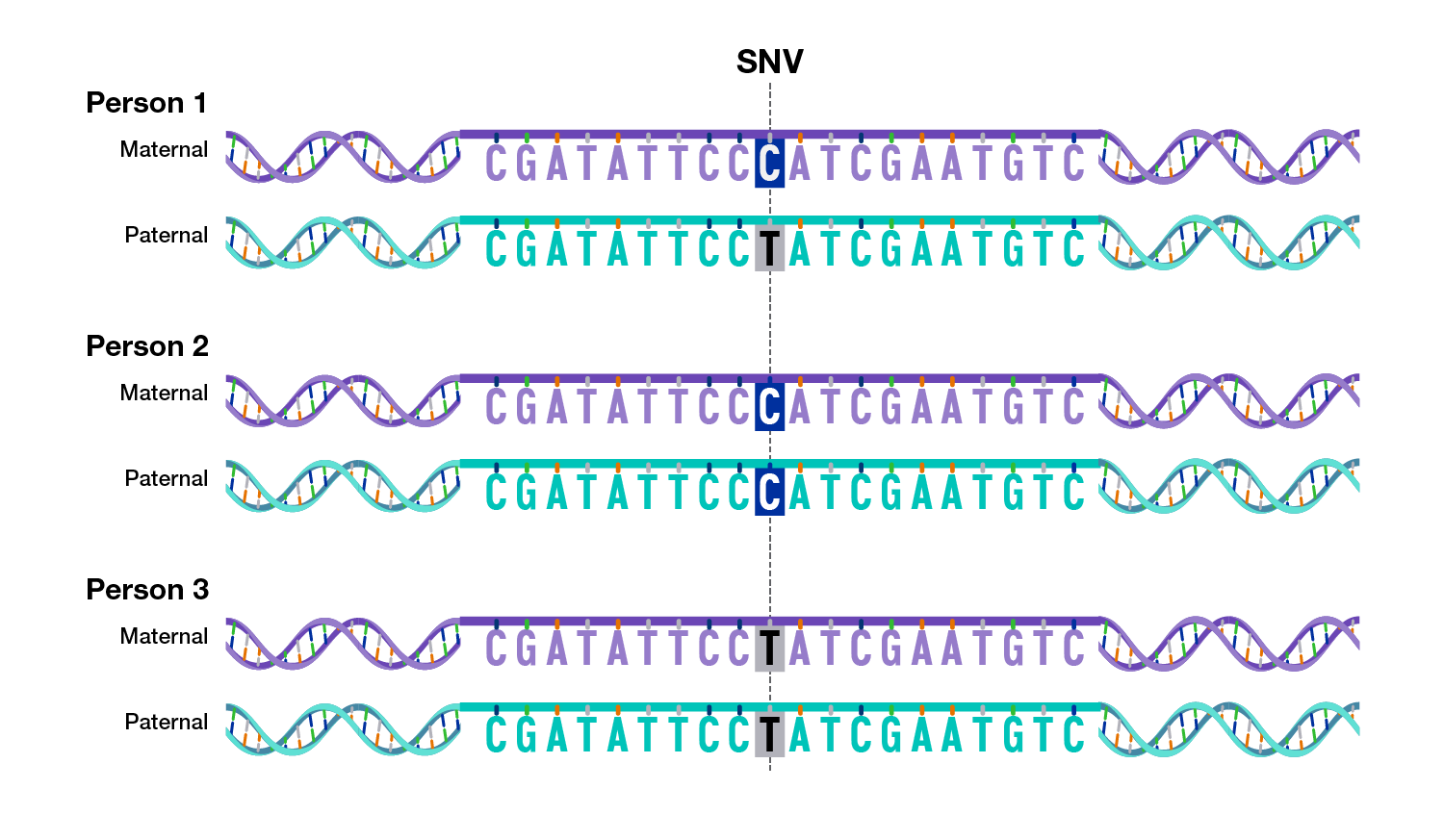
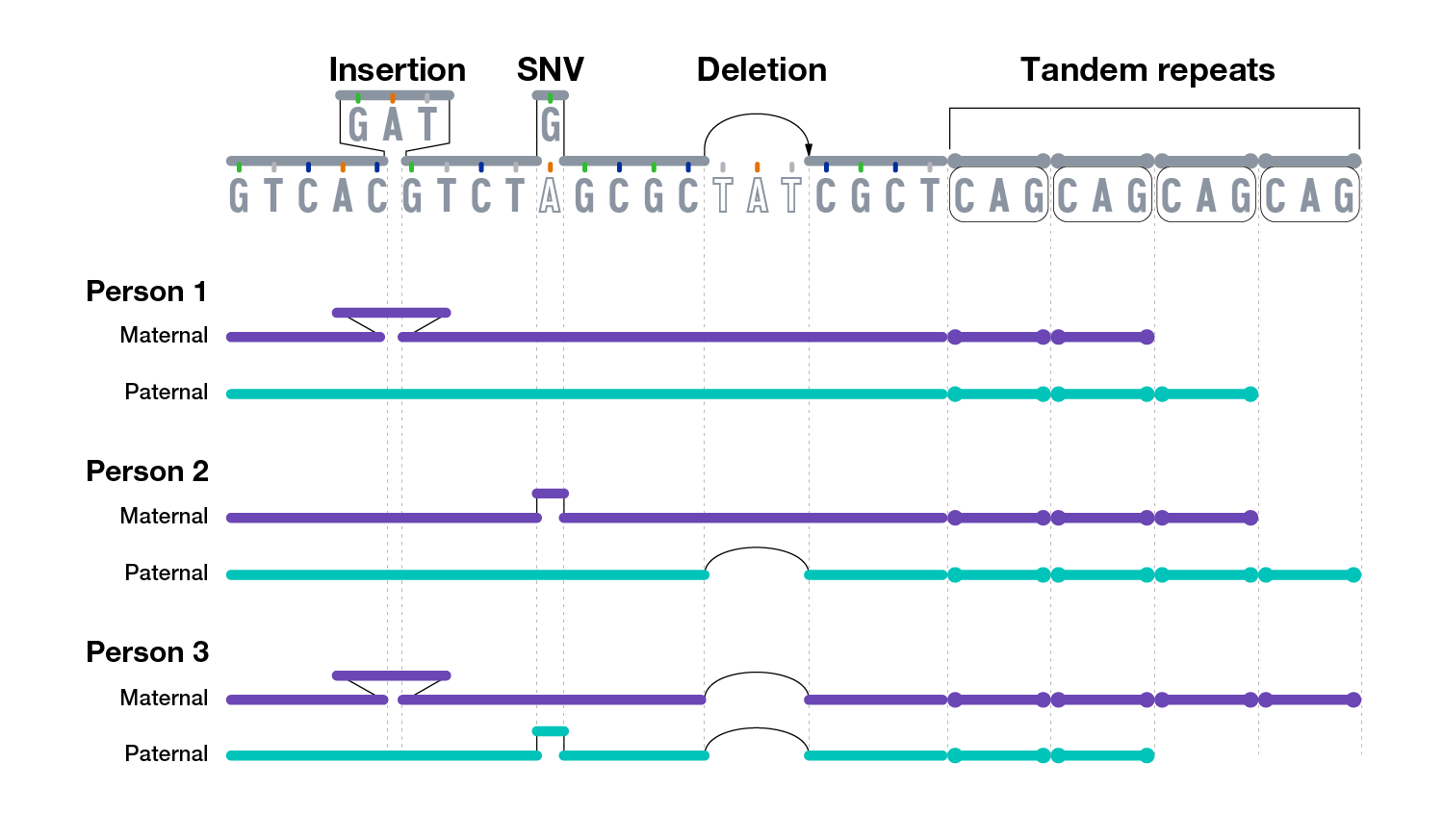
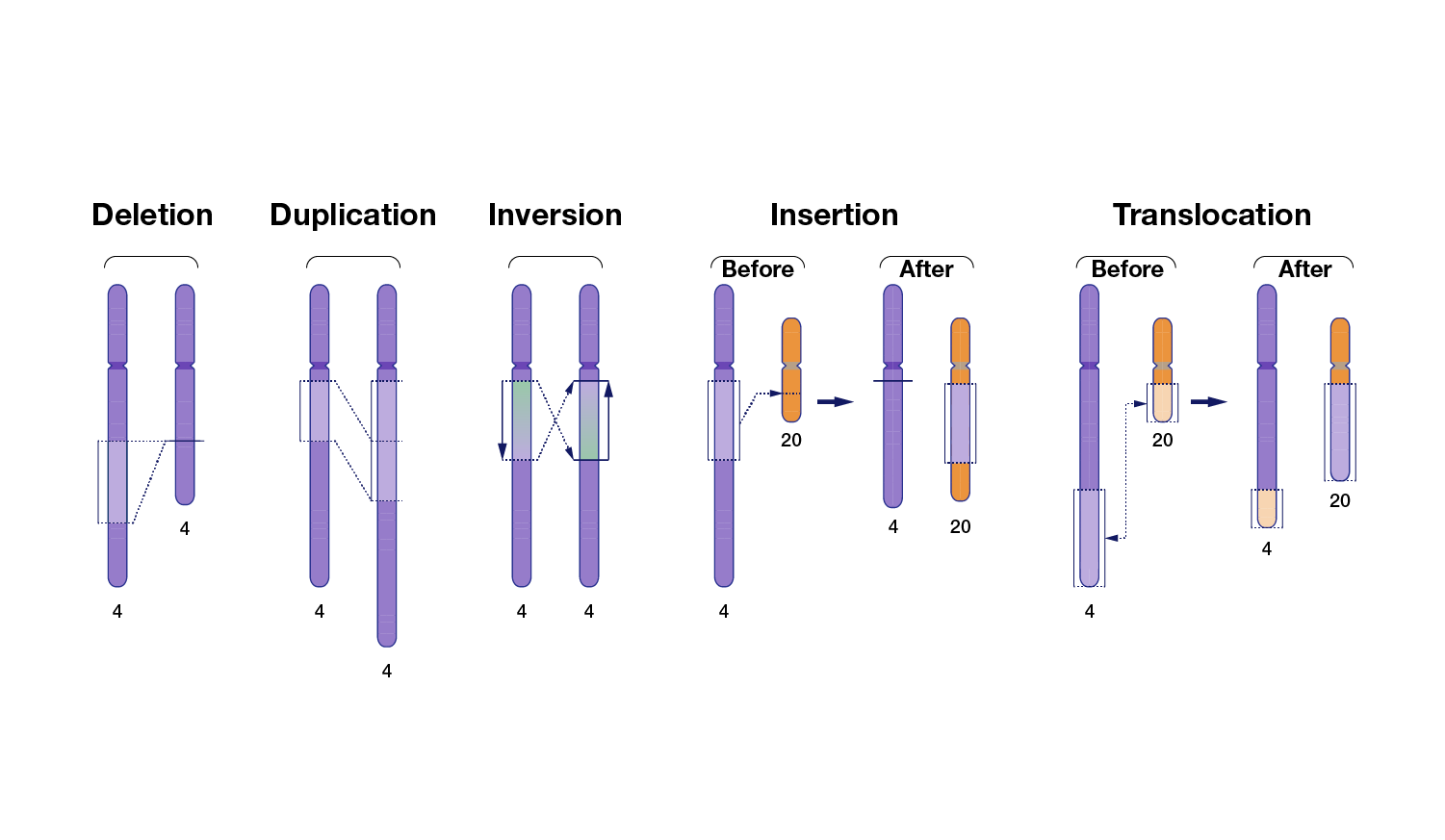
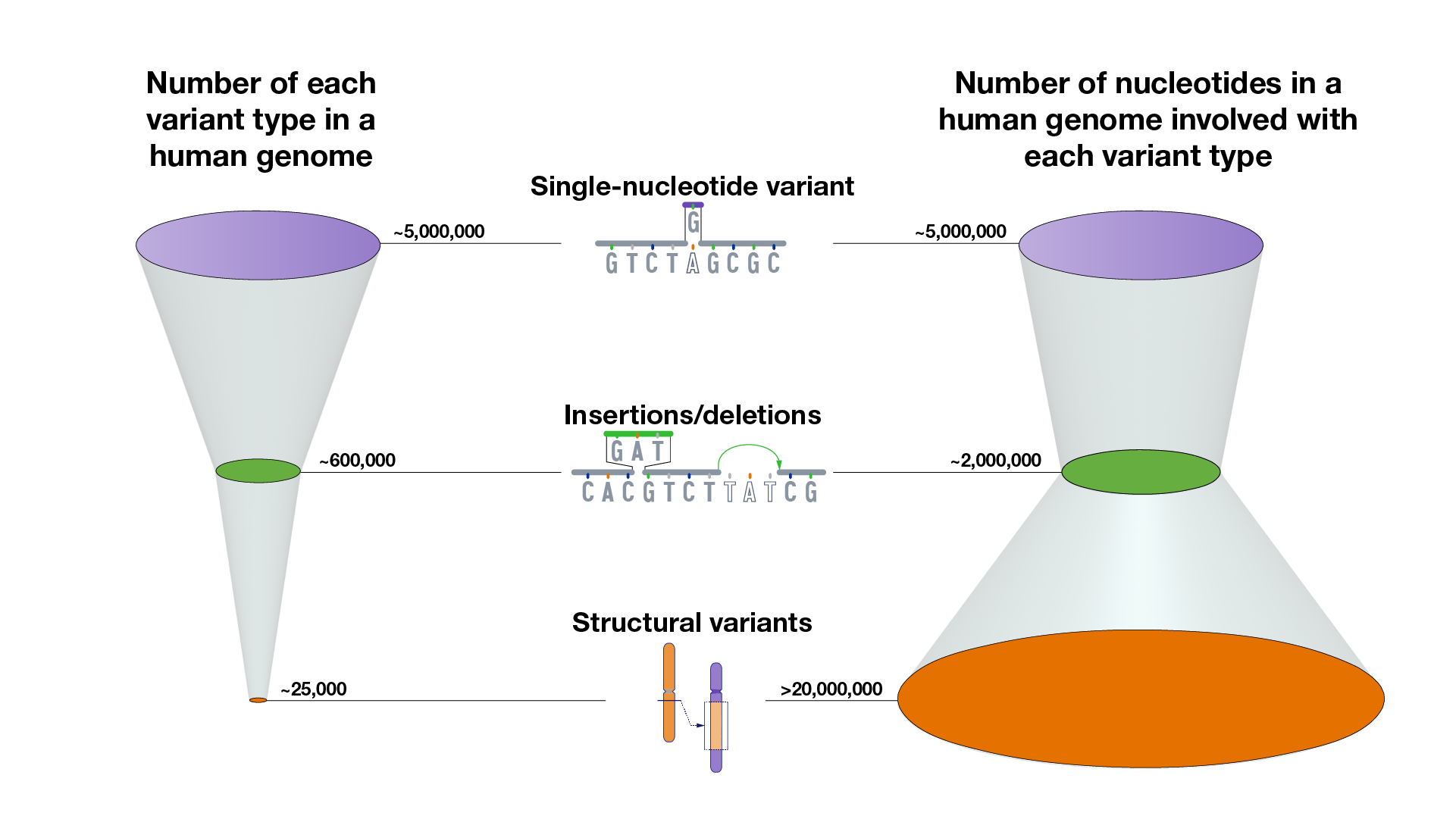
There are multiple types of variants in human genomes, ranging from small differences to large differences.
-
A very small subset of genomic variants contributes to human health and disease.
-
Researchers create reference human genome sequences to help detect genomic variants in each sequenced human genome.
-
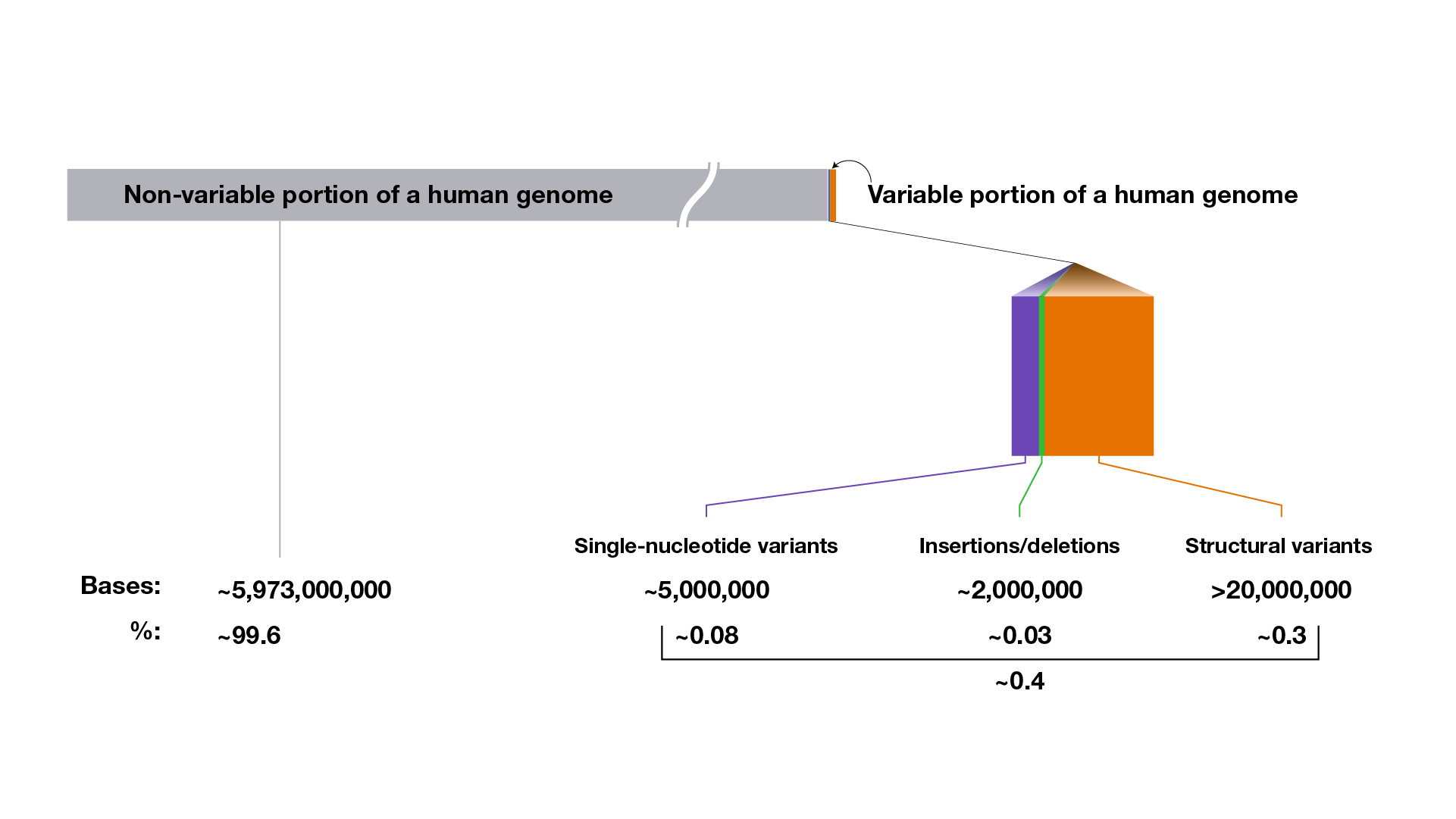
On average, a person’s genome sequence is ~99.6% identical to a reference human genome sequence; that person’s set of genomic variants accounts for the ~0.4% difference.
-
The human pangenome is a more comprehensive framework that aims to account for genomic variation across human populations, thereby reducing biases that can come with the use of a single reference human genome sequence.
Looking forward
Genomics is constantly evolving and advancing, including the regular development of new experimental technologies and data-analysis methods. Such advances are readily applicable to ongoing efforts to develop new and more inclusive sets of reference human genome sequences and improve the ability to detect all variants in each newly sequenced human genome. The long-term goal is to have sufficient knowledge about genomic variation in all human populations, bringing equity in the benefits of genomic medicine.

Last updated: February 1, 2023