Cynthia Tifft, M.D., Ph.D.
Deputy Clinical Director
Office Of The Clinical Director
Senior Clinician
Medical Genetics Branch
Head
Glycosphingolipid and Glycoprotein Disorders Unit
Education
B.A. University of California, San Diego
M.S. Rutgers University
Ph.D. University of Texas, Houston
M.D. University of Texas, Houston
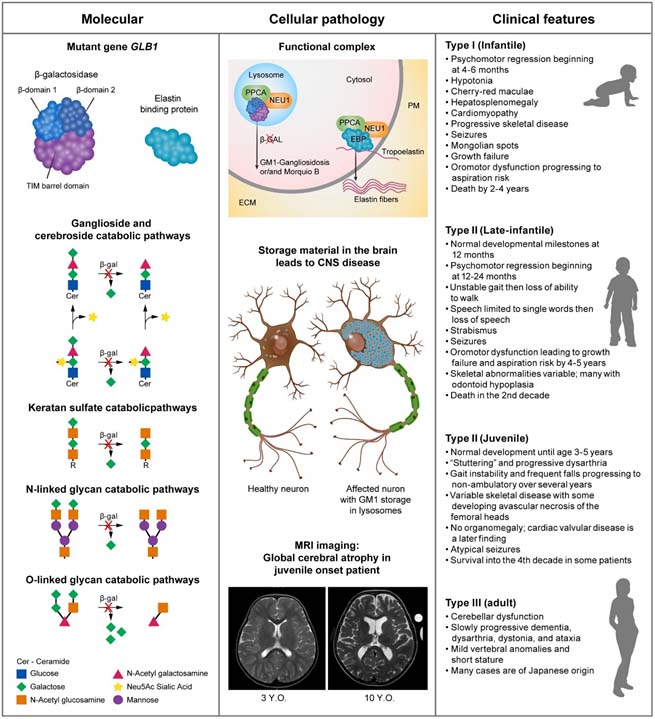
GM1 Gangliosidosis
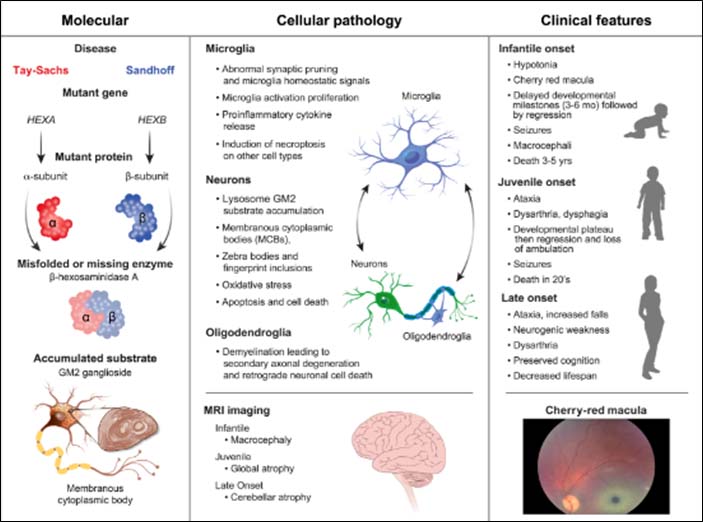
Toro C, Zainab M, Tifft CJ. The GM2 gangliosidoses: Unlocking the mysteries of pathogenesis and treatment. Neurosci Lett. 2021 Nov 1;764:136195. doi: 10.1016/j.neulet.2021.136195. Epub 2021 Aug 25. PMID: 34450229; PMCID: PMC8572160.
GM2 Gangliosidosis
Nicoli ER, Annunziata I, d'Azzo A, Platt FM, Tifft CJ, Stepien KM. GM1 Gangliosidosis-A Mini-Review. Front Genet. 2021 Sep 3;12:734878. doi: 10.3389/fgene.2021.734878. PMID: 34539759; PMCID: PMC8446533.
Glycosphingolipid Disorders and Glycoprotein Disorders Unit

Jean M. Johnston
- Research Nurse Coordinator
- Office of the Clinical Director

Precilla D'Souza, CRNP, DNP
- Pediatric Nurse Practitioner
- NIH Undiagnosed Diseases Program

Connor Lewis, B.S.
- Postbaccalaureate Fellow
- Glycosphingolipid and Glycoprotein Disorders Unit

Mark A. Moran
- Postbaccalaureate Fellow
- Glycosphingolipid Disorders and Glycoprotein Disorders Unit
Last updated: January 12, 2025